Immersion freezing effect on myofibrillar protein characteristics of prepared grass carp (Ctenopharyngodon idellus) fillets during frozen storage
-
摘要:
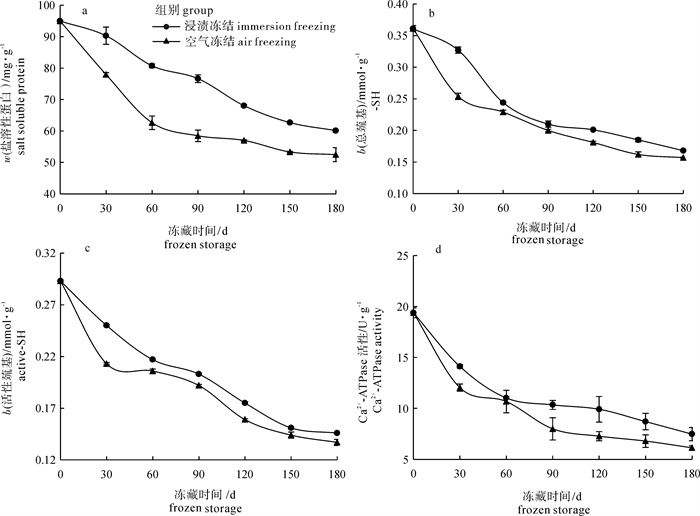
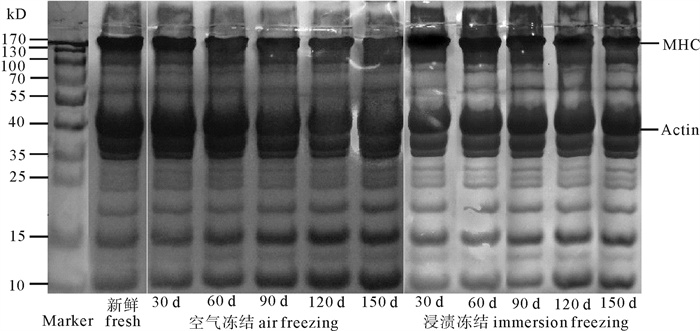
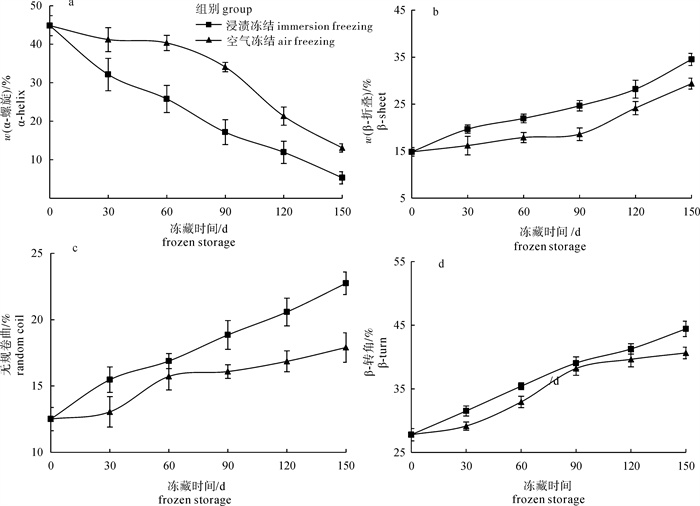
为了探讨浸渍冻结对草鱼(Ctenopharyngodon idellus)冻藏过程蛋白质变性的影响, 对蛋白质溶解性、巯基、Ca2+-ATPase活性、肌原纤维的降解及二级结构进行了研究。结果发现浸渍冻结的肌原纤维蛋白的溶解度、总巯基含量、活性巯基总量和Ca2+-ATPase的活性均比空气冻结的高, 冻藏150 d后分别比空气冻结的高17.51%、14.20%、4.86%和28.73%。初步表明浸渍冻结更有利于减缓蛋白质变性。电泳结果表明, 冻藏过程中浸渍冻结的肌球蛋白重链的电泳条带颜色比空气冻结的深, 原肌球蛋白的电泳条带颜色比空气冻结的浅, 说明浸渍冻结可减缓调理草鱼块蛋白质的降解。红外光谱的结果进一步发现浸渍冻结的肌原纤维蛋白的α-螺旋含量的下降程度较空气冻结的小, β-折叠、无规卷曲和β-转角含量的增加程度无空气冻结的明显。结果揭示了浸渍冻结更有利于维持冻藏过程中肌原纤维蛋白的二级结构。总的来说, 浸渍冻结更有利于减轻蛋白质变性而引起调理草鱼块品质下降的问题。
Abstract:To investigate immersion freezing (IF) effect on protein denaturation of prepared grass carp fillets (PGCFs) during frozen storage, we investigated the protein solubility, sulfhydryl of myofibrillar protein, Ca2+-ATPase activity, degradation of myofibril and secondary structure of myofibril. The results show that the solubility of myofibrillar protein, contents of total sulfhydryl and active sulfhydryl, activity of Ca2+-ATPase of PGCFs by using ICF were higher than those by using air freezing (AF). After frozen storage for 150 d, the contents of protein solubility, total sulfhydryl, active sulfhydryl and Ca2+-ATPase activity of PGCFs by using IF were increased by 17.51%, 14.20%, 4.86% and 28.73% than those by using AF, respectively. It is showed that IF was more advantageous to slow denaturation of protein. Moreover, the electrophoresis result show that the color of heavy chain from myosin of PGCFs by IF was deeper than that by AF, and the color of tropomyosin protein stripe was shallower than that by AF during frozen storage. Thus, IF reduced degradation of protein. The research of infrared spectrum shows that the decreased degree of α-helix content from PGCFs by using IF was less than that by using SAF. Conversely, the increase degree of β-fold, random coil and β-turn from PGCFs by using SAF were more obvious than that by using IF, which indicates that IF was more conducive to maintaining stability of secondary structure of myofibrillar protein during frozen storage. In general, IF was more beneficial to reducing the quality degradation of PGCFs due to protein denaturation.
-
Keywords:
- prepared grass carp /
- immersion freezing /
- myofibrillar protein /
- frozen denaturation
-
人工鱼礁是人为在海中设置的构造物,为鱼类等生物提供繁殖、觅食和躲避敌害的场所,在渔业资源的增殖和海洋生态环境的修复等方面发挥了很好的作用,取得了良好的效果[1-6]。在人工鱼礁的建设过程中,礁区的科学选址和礁体的科学设计是保证人工鱼礁发挥修复和调控作用的首要前提,特别是由于人工鱼礁投放后会受到水流和波浪的冲击,如冲击力过大,礁体可能会发生翻滚、倾覆甚至漂移导致其失效,因此鱼礁设计时的安全性评估是必需的。其中人工鱼礁与海底间的最大静摩擦系数是进行人工鱼礁安全性评估所需要的重要参数[7-11]。
在人工鱼礁稳定性的现有研究中,最大静摩擦系数大多采用的是经验值[12-15]。但经验值并不适用于所有的工况,大量研究表明摩擦系数并不是固定的材料属性,会随外部负载、接触材料特性等条件而发生显著变化。在一个摩擦系统中,只有当材料和工况都确定时,相接触物体间的摩擦系数才能确定[16-19]。因此应首先通过物理模型试验来确定常见材料和工况条件下的最大静摩擦系数,并在此基础上综合分析各因素对最大静摩擦系数的影响规律,进而优化人工鱼礁礁体设计以提高其安全性。近年来,研究人员已逐步开始对人工鱼礁的最大静摩擦系数进行相关的物理模型试验,并在试验中引入了底质粒径、礁体质量等不同的影响因子,以更准确地获得人工鱼礁与海底间的最大静摩擦系数。刘同渝等[20]测量了人工鱼礁在泥质、沙质和石质底质上的最大静摩擦系数,为鱼礁在不同底质上的最大静摩擦系数经验值的选择提供了参考;刘健等[21]研究了底质粒径、礁体质量和开口比大小对最大静摩擦系数的影响规律,并对钢制四方台型鱼礁和十字型鱼礁最大静摩擦系数取值的合理性进行验证;郑延璇等[22]测量了三角型人工鱼礁在细砾和中砂底质上的最大静摩擦系数,用于该礁型的稳定性校核计算。
已有研究表明,进行人工鱼礁最大静摩擦系数物理模型试验时,引入的影响因子越全面,所得到的结果越准确。人工鱼礁与海洋底质构成的摩擦体系中的主要可变因子包括底质特性(底质粒径、含水率)和礁体特征(质量、底面开口比、开口位置),由于在以往的研究中尚未见到关于底质含水率和礁体开口位置对人工鱼礁最大静摩擦系数影响的分析,为了更科学地确定人工鱼礁最大静摩擦系数,文章通过平面拉动法测量研究了6种不同礁型在5种不同粒径(砾石、粗砂、中砂、细砂、粉砂黏土)底质上的最大静摩擦系数,分析了礁体与海底间最大静摩擦系数的5种影响因素(底质粒径、含水率、加载质量、底面开口比、开口位置)及变化规律,以期为人工鱼礁的设计和人工鱼礁区的选址等提供科学依据。
1. 材料与方法
1.1 礁体模型
由于人工鱼礁与海底间的摩擦作用出现在礁体底面与海底泥沙表面间,因此本试验将模型礁体的形状简化为人工鱼礁底面形状。即模型礁体为钢筋混凝土制的长方体结构,底面为正方形[21]。试验中所采用的6个礁体模型见图1,模型的几何尺寸和参数见表1。
表 1 礁体类型与规格Table 1. Type and size of reef models礁体模型
reef model规格/cm
size材质
material孔边长/cm
hole length质量/kg
quality底面积/m2
bottom area开口面积/m2
hole area开口比
opening ratio开口位置
opening position1号 No.1 40×40×5 钢筋混凝土 0 21.86 0.16 0 0 无 2号 No.2 10 21.30 0.010 0 0.06 中心 3号 No.3 15 19.67 0.022 5 0.14 中心 4号 No.4 40×40×5 钢筋混凝土 20 17.50 0.16 0.040 0 0.25 中心 5号 No.5 10 17.50 0.040 0 0.25 1/4中心 6号 No.6 20×10 17.50 0.040 0 0.25 角部 1.2 试验装置
本试验采用平面拉动法测量最大静摩擦系数,试验装置见图2[23],包括一平面承载台、承载台上放置的为不透水有机玻璃盒(图3)。盒内是泥沙和水的混合物,泥沙面高为18.5 cm,水面高为19 cm。礁体模型放置在泥沙层上,平面承载台的一端设有支架,支架上装有滑轮,负载桶和礁体模型之间通过嵌在滑轮轮轴上的钢丝线连接,试验过程中向负载桶内缓慢地加入细砂,至礁体模型有滑动倾向时测量负载桶的质量。
1.3 试验方案
本试验共设计了6种礁型,在5种底质和5种配重的条件下,开展了150组试验。试验前首先采用63 μm、200 μm、500 μm和2 000 μm孔径的分隔网筛分选试验底质泥沙,筛选出5种不同粒径的颗粒:砾石(中值粒径d> mm)、粗砂(0.5 mm<d<2 mm)、中砂(0.2 mm<d<0.5 mm)、细砂(0.063 mm<d<0.2 mm)、粉砂黏土(d<0.063 mm),并向有机玻璃盒中注入淡水至水面高出泥沙面0.5 cm,使泥沙空隙间充满水。
每组试验均采用以下的步骤测量礁体与底质间的最大静摩擦系数:1)测量礁体模型浸水后的质量G;2)将礁体模型轻放在保持水平状态的泥沙层表面,并待其达到静平衡状态;3)用钢丝线水平连接负载桶和礁体模型;4)通过滴漏装置向负载桶内均匀缓慢地加入细砂,使拉动礁体的力均匀缓慢地增加,待观测到礁体有滑动倾向时,立即停止加载并测量负载桶的质量F;5)重复测量5次F,取其平均值
$\overline F$ ;6)在礁体模型上加载2 kg、4 kg、6 kg和8 kg的配重,重复步骤1~5;7)测量试验中所选用的钢丝线和滑轮组的机械效率η。完成所有试验步骤后,根据最大静摩擦系数的计算公式$\mu = \overline F \cdot \eta /G$ ,即可求得该礁体与各组不同粒径底质间的最大静摩擦系数μ。由于本试验中实心礁体的浮力为7.84 N,仅为礁体自重的3%,因此在结果分析中未考虑浮力的作用。本文在第一组试验结果的基础上,为进一步研究含水率对粉砂黏土底质上礁体最大静摩擦系数的影响,配制了7种不同含水率的粉砂黏土土样。配制过程中首先称取8 kg烘干后的粉砂黏土土样,加入1.6 kg的淡水充分搅拌混合,配制成含水率为20%的粉砂黏土土样用于试验,在每次试验的基础上分别加入0.4 kg的淡水,依次配制成25%、30%、35%、40%、45%和50%含水率的粉砂黏土土样用于试验(图4)。
由于含水率较大时土样为流塑状态,压缩性大,承载力小,礁体放置于其表面极易沉陷,所以选用质量为0.4 kg的圆形实心礁体按照上述的步骤进行试验(图5)。
1.4 数据处理
根据试验数据,采用Origin 9.1软件做出曲线图并分析各因素之间的关系,采用专业统计软件SPSS 19.0对最大静摩擦系数与泥沙粒径、加载质量、开口比及开口位置进行相关性分析[24],其中P<0.01为极显著性水平,P<0.05为显著性水平,P>0.05为无显著性水平。
2. 结果与讨论
2.1 底质泥沙粒径与最大静摩擦系数的关系
本组试验中礁体加载质量均为0,各底质均处于饱和含水率的状态,每种模型进行多次试验后,取平均值为最大静摩擦系数。当底质分别为粉砂黏土、细砂、中砂、粗砂和砾石时,各礁体相对应的最大静摩擦系数为0.10、0.61、0.60、0.58和0.53 (图6)。底质粒径大于0.063 mm时最大静摩擦系数随底质粒径增大而减小。
进一步对细砂、中砂、粗砂和砾石共4种底质条件下最大静摩擦系数与泥沙粒径的相关性进行分析,得到相关系数为R=−0.858**,表明在0.01水平检验下(双侧)最大静摩擦系数与泥沙粒径显著负相关。本试验结果与已有的研究一致[20-22],这主要是因为最大静摩擦系数与实际接触面积成正比,随着泥沙粒径的减小,泥沙的密实度增大,进而增加了泥沙与礁体的实际接触面积,最大静摩擦系数也随之增大[25]。但当底质为粉砂黏土时,最大静摩擦系数大大减小,与刘健等[21]测量的数值存在差异,其主要原因是粉砂黏土中细粒含量和细粒含水率对最大静摩擦系数的影响很大,当细粒含量大并且含水较多时,细粒处于流塑状态,在挤压过程中起到润滑作用,从而降低最大静摩擦系数[26-28]。因此在人工鱼礁区选址时,应尽量选择海床表层为细砂或粗砂的海域,避免选择海床表层为厚粉砂黏土层的海域,以确保人工鱼礁达到理想的养护增殖效果。
2.2 含水率与粉砂黏土底质上礁体的最大静摩擦系数的关系
由于第一组试验的结果表明,含水率对粉砂黏土底质条件下的人工鱼礁最大静摩擦系数有明显影响,为进一步研究含水率的影响规律,本组试验在7种不同含水率的粉砂黏土底质上测量得出最大静摩擦系数与含水率的关系见图7。
试验结果显示,礁体与粉砂黏土底质间的最大静摩擦系数随含水率的增加而增加,当含水率增至35%时最大静摩擦系数达到最大值,此后,随着含水率的继续增大,最大静摩擦系数又迅速减小(图7)。出现这种变化特征的主要原因是在含水率由小变大的过程中,黏土中越来越多的弱结合水逐渐吸附在强结合水外围,增加了土粒外围结合水的分子数量,加大了土粒间的黏聚力,增加了礁体与泥沙间的外摩擦系数。但当含水率超过一定值时,黏土中的弱结合水形成较厚的结合水膜,增大了土粒间的距离,导致黏聚力减弱。同时,过量的水还起到润滑作用,使外摩擦系数减小[29-30]。本组试验表明,当建礁海域底质表层为粉砂黏土时,底质含水率是影响人工鱼礁最大静摩擦系数的重要因素,因此在开展人工鱼礁区选址调查时,不仅应对底质粒径进行采样分析,还需分析底质含水率,并结合含水率对人工鱼礁的稳定性进行校核。
2.3 加载质量与最大静摩擦系数的关系
本组试验设计了5组加载质量条件,对各底质条件下礁体受到不同载荷时的最大静摩擦系数进行了测量。当底质为砂质、砾石和粉砂黏土时,摩擦系数均随垂直载荷的增加而逐渐减小(图8)。其中1号~3号礁体在各底质上的最大静摩擦系数随加载质量增加而下降的趋势较明显,变化幅度为4%~7%;4号~6号礁体在细砂和粉砂黏土底质上随加载质量变化时,最大静摩擦系数变化趋势平缓,变化幅度小于3%;5号和6号礁体在粗砂底质上最大静摩擦系数随加载质量增加而下降的幅度达到8%。
试验发现最大静摩擦系数随着法向载荷质量的增加而逐渐减小,本结果与以往研究结果一致[31-33]。主要原因是当载荷增加时,泥沙密实度增大进而导致真实接触面积增大,但真实接触面积的增幅小于载荷的增幅,且润滑条件得到改善,多种因素最终导致摩擦系数减小。此外,较大的压力也会限制前文所提到的黏聚力,减小了摩擦力[29]。尽管研究表明最大静摩擦系数与加载质量呈负相关关系,且自重过大容易造成礁体沉降,但设计中根据投放礁体的海域底质特点,可通过力学分析确定礁体的最优设计质量,既避免严重沉降,又增加礁体与底质间的最大静摩擦力,使礁体投放在海底后更加稳定。
2.4 礁体开口比与最大静摩擦系数的关系
礁体开口比是礁体底面开口面积与底面积之比,是一个礁体底面与海床接触面积的表征参数。通常来说,开口比越大的礁体越容易下陷,礁体所受的摩擦阻力就越大。本组试验测量比较了开口比分别为0.00 (1号礁)、0.06 (2号礁)、0.14 (3号礁)、0.25 (4号礁)的4种礁型在5种粒径底质上的最大静摩擦系数(图9),以加载质量为0 kg和2 kg为例,当底质为中砂、粗砂、砾石时,随着开口比的增大,最大静摩擦系数有相对明显的增加趋势;而当底质为细砂和粉砂黏土时,最大静摩擦系数的变化趋势不明显。
不同开口比的礁体其质量也不同,因此试验所得最大静摩擦系数的变化趋势是受到加载质量和开口比2种因素的影响。为进一步分析开口比的变化对最大静摩擦系数的影响,需控制加载质量,对开口比进行偏相关性分析(表2)。最大静摩擦系数与加载质量是显著负相关关系,与开口比是显著正相关关系,且加载质量对最大静摩擦系数的影响程度比开口比要大,其主要原因是当礁体质量一定时,开口比越大,礁体底部单位面积上的受力越大,底部泥沙密实度随之增大最后导致摩擦系数增大。目前对人工鱼礁底面开口比的设计通常以防止礁体沉降为主,本试验结果揭示了开口比的优化可提升人工鱼礁的防滑移能力,因此在控制礁体沉降之外适当增加人工鱼礁的底部开口比,既能增强人工鱼礁的稳定性,又能减少其制作成本。
表 2 偏相关分析系数Table 2. Coefficients of partial correlation analysis粉砂黏土
silty clay细砂
fine sand中砂
medium sand粗砂
coarse sand砾石
gravel加载质量 loading quality R=−0.919
P=0R=−0.858
P=0R=−0.937
P=0R=−0.664
P=0.002R=−0.798
P=0开口比 opening ratio R=0.573
P=0.01R=−0.204
P=0.402R=0.653
P=0.002R=0.651
P=0.003R=0.656
P=0.002注:R. 相关系数;P. 不相关概率
Note: R. correlation coefficient;P. uncorrelated probability2.5 礁体开口位置与最大静摩擦系数的关系
由于礁体底面的设计各有不同,因此有必要对开口面积相同,但开口位置不同的礁体进行不同底质条件下的静摩擦试验。以2 kg加载质量为例,当开口面积一定时,最大静摩擦系数几乎不随开口位置的变化而变化(图10)。通过控制底质泥沙粒径,对开口位置进行单因素方差分析,得到结果为P=0.996>0.05,说明人工鱼礁底面的开口位置对最大静摩擦系数的变化无显著性影响。因此,在开口比的设计上应遵循以下原则:1)开口比例适中,既具有一定的防沉降能力,又可适当增大最大静摩擦力;2)采用相对简单的制作工艺,利于施工又可降低人工鱼礁建设成本。
3. 结语
本文通过物理模型试验,系统研究了底质粒径、底质含水率、加载质量、人工鱼礁底面开口比特征等因素对人工鱼礁静摩擦系数的影响,得到了以下主要结论:1)海床表层底质主成分为砂质的海域总体上会更利于人工鱼礁投放后的稳定性,而从海床表层为粉砂黏土时的试验结果看,粉砂黏土底质含水率对人工鱼礁静摩擦系数有很大影响,结合以往的相关研究[34-36],其厚度及含水率垂直方向上的变化特征应作为人工鱼礁设计中考虑的重要因素,在进行人工鱼礁区选址时,不仅应调查底质特征,还应掌握底质含水率随深度的变化,进而根据投礁区底质的承载力和礁体的自重算出礁体的下陷深度,结合该深度对应的含水率,通过模型试验确定最大静摩擦系数,在此基础上再对人工鱼礁进行安全校核,以保证人工鱼礁建设效果;2)礁体自重和底面开口比均对最大静摩擦系数产生了影响,开口位置造成的影响可忽略不计,因此在工程实践中应结合相关试验结果对礁体结构进行优化,在确保工程质量的前提下有效降低建设成本。
由于受到条件的限制,本试验仍有一定局限性,如未能考虑海水的冲刷、礁体投放后的初始姿态及不同海床坡度等因素,此外在对各影响因素的权重分析方面也存在不足,在后续的研究工作中应进一步完善试验设计和数据分析,为人工鱼礁设计的最优化和建设效果的最大化提供科学依据。
-
-
[1] 任丽娜. 白鲢鱼肉肌原纤维蛋白冷冻变性的研究[D]. 无锡: 江南大学, 2014: 23. https://xueshu.baidu.com/usercenter/paper/show?paperid=3d69653d080434b84572e980faf88c87&site=xueshu_se&hitarticle=1 [2] BENJAKUL S, VISESSANGUAN W, THONGKAEW C, et al. Comparative study on physicochemical changes of muscle proteins from some tropical fish during frozen storage[J]. Food Res Int, 2003, 36(8): 787-795. doi: 10.1016/S0963-9969(03)00073-5
[3] 吴光红, 史婷华. 淡水鱼糜的特性[J]. 上海水产大学学报, 1999, 8(2): 154-162. [4] 刘艺杰. 鳙鱼(Aristichthys nobilis)和秘鲁鱿鱼(Dosidicus gigas)肌肉蛋白质理化特性及凝胶特性的研究[D]. 青岛: 中国海洋大学, 2006: 32. https://xueshu.baidu.com/usercenter/paper/show?paperid=f26a92ab7288b06e112c02445bd2beff&site=xueshu_se&hitarticle=1 [5] 农业部渔业渔政管理局. 中国渔业统计年鉴[M]. 北京: 中国农业出版社, 2014: 23-43. [6] LIN W L, ZENG Q X, ZHU Z W. Relation between protein characteristics and TPA texture characteristics of crisp grass carp (Ctenopharyngodon idellus C. et V) and grass carp (Ctenopharyngodon idellus)[J]. J Texture Stud, 2012, 43(1): 1-11. doi: 10.1111/j.1745-4603.2011.00311.x
[7] 鲁长新. 淡水鱼肌肉的热特性研究[D]. 武汉: 华中农业大学, 2007: 19. https://xueshu.baidu.com/usercenter/paper/show?paperid=9a826a9d138327c1a3ad76d9943069ad&site=xueshu_se&hitarticle=1 [8] 关磊, 朱瑞俊, 李小勤, 等. 普通草鱼与脆化草鱼的肌肉特性比较[J]. 上海海洋大学学报, 2011, 20(5): 748-753. https://xueshu.baidu.com/usercenter/paper/show?paperid=da6ae768e795bdfdda21332bcb854f10&site=xueshu_se&hitarticle=1 [9] 林婉玲, 杨贤庆, 宋莹, 等. 浸渍冻结对调理草鱼块冻藏过程中品质的影响[J]. 现代食品科技, 2014, 30(10): 80-87. https://xueshu.baidu.com/usercenter/paper/show?paperid=d501483db7a026e1cbcbbe9966060ce5&site=xueshu_se&hitarticle=1 [10] 杨贤庆, 宋莹, 林婉玲, 等. 草鱼调理食品加工工艺[J]. 食品与发酵工业, 2014, 40(1): 101-106. https://xueshu.baidu.com/usercenter/paper/show?paperid=59e244fda91e330f33af29d8f4a21137&site=xueshu_se&hitarticle=1 [11] 张琼, 章梁, 黄泽元. 草鱼鱼片热风干燥特性的研究[J]. 武汉工业学院学报, 2008, 27(4): 13-18. doi: 10.3969/j.issn.1009-4881.2008.04.004 [12] 胡王, 陈小雷, 李海洋, 等. 茶香即食草鱼的工艺及产品特性研究[J]. 安徽农业科学, 2014, 42(5): 1517-1519. doi: 10.3969/j.issn.0517-6611.2014.05.085 [13] 李新, 耿胜荣, 汪兰, 等. 即食风味草鱼的开发与灭菌[J]. 辐射研究与辐射工艺学报, 2014, 32(1): 60-66. https://xueshu.baidu.com/usercenter/paper/show?paperid=bf15f74ef52fedfa203f3b88517fe07a&site=xueshu_se&hitarticle=1 [14] 欧阳杰, 谈佳玉, 沈建, 等. 浸渍冻结大黄鱼贮藏期间品质变化研究[J]. 南方水产科学, 2013, 9(6): 72-77. doi: 10.3969/j.issn.2095-0780.2013.06.012 [15] 邹明辉. 无磷保水剂在凡纳滨对虾虾仁冻藏加工中的应用及保水机理研究[D]. 湛江: 广东海洋大学, 2011: 39-40. https://xueshu.baidu.com/usercenter/paper/show?paperid=fd046e7f562fde2a9a2a2c3c0261ae54&site=xueshu_se&hitarticle=1 [16] 吴燕燕, 游刚, 李来好, 等. 无磷品质改良剂对阿根廷鱿鱼冷冻变性的影响[J]. 南方水产科学, 2013, 9(5): 19-24. doi: 10.3969/j.issn.2095-0780.2013.05.004 [17] 曾名勇, 黄海, 李八方. 鳙肌肉蛋白质生化特性在冻藏过程中的变化[J]. 水产学报, 2003, 27(5): 480-485. https://xueshu.baidu.com/usercenter/paper/show?paperid=1b130pp0mk1y0xn01j4t0my0qy697627&site=xueshu_se&hitarticle=1 [18] ETEMADIAN Y, SHABANPOUR B, MAHOONAK A, et al. Cryoprotective effects of polyphosphates on Rutilus frisii kutum fillets during ice storage[J]. Food Chem, 2011, 129(4): 1544-1551. doi: 10.1016/j.foodchem.2011.06.005
[19] SOMPONGSE I Y, OBATAKE A. Effect of cryoprotectants and a reducing reagent on the stability of actomyosin during ice storage[J]. Fish Sci, 1996, 62(1): 73-79. doi: 10.2331/fishsci.62.73
[20] 郭园园, 孔保华. 冷冻贮藏引起的鱼肉蛋白质变性及物理化学特性的变化[J]. 食品科学, 2011, 32(7): 335-340. https://xueshu.baidu.com/usercenter/paper/show?paperid=466c4b384280682429535fc6ae0defa0&site=xueshu_se&hitarticle=1 [21] BADⅡ F, HOWELL N K. Changes in the texture and structure of cod and haddock fillets during frozen storage[J]. Food Hydrocolloids, 2002, 16(4): 313-319. doi: 10.1016/S0268-005X(01)00104-7
[22] 何建川, 邵阳, 张波. 蛋白质和变性蛋白质二级结构的FTIR分析进展[J]. 化学研究与应用, 2012, 24(8): 1176-1180. doi: 10.3969/j.issn.1004-1656.2012.08.002 [23] 钟朝辉, 李春美, 顾海峰, 等. 温度对鱼鳞胶原蛋白二级结构的影响[J]. 光谱学与光谱分析, 2007, 27(10): 1970-1976. https://xueshu.baidu.com/usercenter/paper/show?paperid=a7feeac961f4e58cb8aa03a62b0d3e19&site=xueshu_se&hitarticle=1 [24] 蔡联辉, 曾虹燕, 蔡西玲, 等. 莲子蛋白组分二级结构的研究[J]. 光谱学与光谱分析, 2011, 31(9): 2394-2398. https://xueshu.baidu.com/usercenter/paper/show?paperid=7b6e3839111679ed5de9ebe0afeea3dc&site=xueshu_se&hitarticle=1 -
期刊类型引用(26)
1. 温利红,张衡,方舟,陈新军. 鸢乌贼渔业资源研究进展. 水产科学. 2023(03): 527-537 .  百度学术
百度学术
2. 招春旭,吴文秀,邱星宇,周倍合,谢嘉仪,康斌,颜云榕. 南海不同海域鸢乌贼生长与死亡参数比较. 上海海洋大学学报. 2021(02): 294-300 .  百度学术
百度学术
3. 谢嘉仪,张丽姿,吴文秀,周倍合,陈秋杰,招春旭,何雄波,徐军,颜云榕. 南沙群岛海域鸢乌贼摄食习性与营养生态位. 水产学报. 2021(12): 1993-2002 .  百度学术
百度学术
4. 陈炫妤,陆化杰,王洪浩,何静茹,刘凯,陈新军. 西北印度洋鸢乌贼角质颚色素沉积特性分析. 动物学杂志. 2020(04): 468-476 .  百度学术
百度学术
5. 朱凯,张立川,肖楚源,陈新军,林东明,朱俊磊. 南海鸢乌贼微型群雌性个体繁殖力研究. 渔业科学进展. 2020(06): 140-148 .  百度学术
百度学术
6. 朱凯,姚吉祥,陈新军,刘维达,孙程婕,林东明. 南海鸢乌贼微型群肌肉和性腺组织能量积累及其分配. 上海海洋大学学报. 2020(06): 910-920 .  百度学术
百度学术
7. 朱凯,王雪辉,杜飞雁,张鹏,邱永松. 南海中南部鸢乌贼中型群与微型群形态指标的分析比较. 中国海洋大学学报(自然科学版). 2019(01): 43-54 .  百度学术
百度学术
8. 李敏,张鹏,张俊,张魁,陈作志. 南海鸢乌贼的遗传差异:种群分化还是种间分化. 中国水产科学. 2019(01): 133-140 .  百度学术
百度学术
9. 朱凯,张立川,陈新军,姚吉祥,韩飞,林东明. 南海鸢乌贼中型群雄性个体肌肉和性腺组织能量积累及其分配. 热带海洋学报. 2019(04): 41-51 .  百度学术
百度学术
10. 朱凯,张立川,陈新军,陆化杰,林东明,姚吉祥,马有成. 基于精荚数量对鸢乌贼中型群雄性个体有效繁殖力的研究. 动物学杂志. 2019(04): 517-528 .  百度学术
百度学术
11. 黄佳兴,龚玉艳,徐姗楠,陈作志,张俊,于文明. 南海中西部海域鸢乌贼中型群和微型群的营养生态位. 应用生态学报. 2019(08): 2822-2828 .  百度学术
百度学术
12. 刘玉,王雪辉,杜飞雁,刘必林,张鹏,刘梦娜,邱永松. 南海鸢乌贼耳石微量元素差异性分析. 南方水产科学. 2019(05): 15-24 .  本站查看
本站查看
13. 朱凯,孙程婕,陈新军,林东明,刘海涛,古昊. 南海鸢乌贼中型群雌性个体肌肉和性腺组织能量积累及其分配. 海洋渔业. 2019(06): 641-651 .  百度学术
百度学术
14. 刘玉,王雪辉,杜飞雁,刘必林,张鹏,刘梦娜,邱永松. 基于耳石微结构的南海鸢乌贼日龄和生长研究. 热带海洋学报. 2019(06): 62-73 .  百度学术
百度学术
15. 龚玉艳,孔啸兰,杨玉滔,詹凤娉,张鹏,江艳娥,陈作志. 南海鸢乌贼微型群体的摄食习性研究. 海洋渔业. 2018(04): 394-403 .  百度学术
百度学术
16. 徐亮,宁加佳,王雪辉,徐磊,刘玉,杜虹,杜飞雁. 脂类去除对南海鸢乌贼肌肉碳、氮稳定同位素分析的影响. 南方水产科学. 2018(04): 88-93 .  本站查看
本站查看
17. 江淼,马胜伟,吴洽儿. 南海鸢乌贼资源探捕与开发. 中国渔业经济. 2018(02): 65-70 .  百度学术
百度学术
18. 粟丽,陈作志,张鹏,李杰,王欢欢,黄佳兴. 2017年南海中南部渔场灯光罩网渔获物组成及渔获率时空分布. 南方水产科学. 2018(05): 11-20 .  本站查看
本站查看
19. 王雪辉,邱永松,张鹏,杜飞雁. Natural mortality estimation and rational exploitation of purpleback flying squid Sthenoteuthis oualaniensis in the southern South China Sea. Chinese Journal of Oceanology and Limnology. 2017(04): 902-911 .  百度学术
百度学术
20. 于鑫,单秀娟,李忠义,朱仁,陈云龙,金显仕. 渤海底拖网主要渔业生物类别时空分布的初步研究. 南方水产科学. 2017(02): 9-17 .  本站查看
本站查看
21. 黄卉,杨丽芝,杨贤庆,李来好,郝淑贤,魏涯,王锦旭. 南海鸢乌贼墨汁多糖分离纯化及组分分析. 食品科学. 2017(24): 118-123 .  百度学术
百度学术
22. 招春旭,陈昭澎,何雄波,邓玉淑,冯波,颜云榕. 基于耳石微结构的南海春季鸢乌贼日龄、生长与种群结构的研究. 水生生物学报. 2017(04): 884-890 .  百度学术
百度学术
23. 朱凯,杜飞雁,王雪辉,邱永松,张鹏. 南海中南部鸢乌贼中型群群体结构. 应用生态学报. 2017(04): 1370-1376 .  百度学术
百度学术
24. 粟丽,陈作志,张鹏. 南海中南部海域春秋季鸢乌贼繁殖生物学特征研究. 南方水产科学. 2016(04): 96-102 .  本站查看
本站查看
25. 龚玉艳,詹凤娉,杨玉滔,张鹏,孔啸兰,江艳娥,陈作志. 南海鸢乌贼摄食习性的初步研究. 南方水产科学. 2016(04): 80-87 .  本站查看
本站查看
26. 朱凯,王雪辉,张鹏,杜飞雁,邱永松. 南海南部鸢乌贼中型群与微型群形态学差异及其判别分析. 热带海洋学报. 2016(06): 82-88 .  百度学术
百度学术
其他类型引用(8)




 下载:
下载:











 粤公网安备 44010502001741号
粤公网安备 44010502001741号
