Optimization of expression conditions for recombinant protein of biomineral gene Pearlin from pearl oyster (Pinctada fucata)
-
摘要:
为了获得稳定高表达的合浦珠母贝(Pinctada fucata)壳基质蛋白Pearlin基因的重组融合蛋白,该研究从合浦珠母贝外套膜总RNA中扩增得到Pearlin的cDNA,将其ORF克隆至原核表达载体pET32a构建得到重组质粒pET32a-Pearlin,并转化表达宿主菌大肠杆菌(E.coli)BL21(DE3),得到的原核重组融合蛋白pET32a-Pearlin约为34.19 kD。对重组融合蛋白表达所需IPTG诱导浓度、培养温度、培养基pH及IPTG诱导时间、时机进行了优化。结果显示,IPTG浓度在0.6~1.4 mmol·L-1范围内诱导效果最佳;在IPTG终浓度为1 mmol·L-1、相同培养时间(6 h)下,37 ℃表达蛋白最多;重组蛋白在pH分别为6.0、7.0、8.0的培养基中表达量变化不大;在IPTG诱导浓度一定的条件下,最佳诱导时间为4~6 h;在IPTG诱导浓度和诱导时间一定的条件下,在转接3~4 h后进行IPTG诱导蛋白表达量较理想。对重组融合蛋白pET32a-Pearlin的可溶性进行检测,发现在不同的诱导条件下,融合蛋白都主要以包涵体形式存在。
Abstract:To obtain high and stable expression recombinant fusion protein of Pearlin, we constructed the recombinant vector by using the open reading frames (ORF) of Pearlin cloned from the mantle tissue of pearl oyster (Pinctada fucata) and optimized the expression conditions. The Pearlin ORF was cloned into the vector pET32a and the plasmids of pET32a-Pearlin were obtained and then transformed into E.coli BL21(DE3). His-tagged insoluble fusion protein was highly expressed and the molecular weight of the fusion protein was about 34.19 kD. We optimized the conditions for IPTG concentrations, induction duration and timing, and temperature and pH of the medium. The results showed that the optimal IPTG concentration ranged from 0.6 mmol·L-1 to 1.4 mmol·L-1 and the best temperature was 37 ℃ when IPTG concentration was 1.0 mmol·L-1 and incubation time was 6 h. The expression levels of recombinant protein did not change significantly when the pH of medium was 6.0, 7.0 and 8.0. When IPTG concentration was kept constant (1.0 mmol·L-1), 4~6 h induction was optimal; when IPTG concentration (1.0 mmol·L-1) and induction duration (6 h) were kept constant, the best starting time for induction was 3~4 h after transformation. Solubility test indicates that fusion protein pET32a-Pearlin was mainly in the form of inclusion body.
-
南海北部资源状况对整个南海渔业的可持续开发至关重要,近年来,由于过度开发和海洋环境变化等因素,南海近海渔业资源严重衰退,呈现优质经济鱼类数量比例下降、种类组成小型化、低龄化、性成熟提早的严峻生物学表现[1]。为了恢复枯竭的近海渔业资源,1999年起中国在南海海域实行伏季休渔,每年6月1日—7月31日,在北纬12°以北的南海海域(含北部湾),禁止所有拖网(含拖虾、拖贝)、围网及掺缯作业。2000年伏季休渔的作业类型扩大为“除刺网、钓业外的其他所有作业类型”[2-3]。2009年伏季休渔的时间延长至两个半月,调整为5月16日—8月1日。2017年南海伏季休渔的时间进一步延长至5月1日—8月16日。至今南海的伏季休渔已连续实施了19年,取得了公认的生态效益、经济效益和社会效益[4-9]。相关研究主要以现场调查和声学调查为主[9-10],伏季休渔生态效应的定量研究还需要多学科交叉和多技术手段的综合应用。卫星遥感提供了从空间快速获取大量海面信息的途径,具有时效性高、客观性强、监测范围大等显著优势,已成功应用于资源评估、环境监测、污染灾害预警预报、海洋工程等领域[11-13]。利用卫星遥感技术开展伏季休渔效果研究,评估当前伏季休渔对渔业资源恢复和养护的作用,对于进一步完善休渔制度和提高渔业资源管理水平具有重要意义。
伏季休渔有效缓解了近海渔业资源衰退的局面、减少了对海域生态环境的破坏,为渔业资源的可持续利用奠定了良好的基础,但也存在对休渔对象和捕捞作业的管理不足等亟待解决的难点问题[4-8]。因此,本研究根据渔业环境卫星遥感资料和现场拖网调查的结果,从海域生态环境和渔业资源生物量变动等方面,评价大亚湾伏季休渔的实施效果,提出完善休渔制度的建议,为南海近岸渔业资源的可持续利用和管理提供科学依据。
1. 材料与方法
1.1 调查方法
休渔前(2015年5月)和休渔后(2015年8月)按照设置的站位对大亚湾海域进行了拖网调查 (图1)。各项监测和采样方法均按《海洋调查规范》(GB 12763—2007)和《海洋监测规范》(GB 17378—2007)规定方法进行。拖网调查船主机功率219.32 kW,总吨位104.98 t,拖网上纲46 m,下纲46 m,网口周长30.7 m,网口网目为4.2 cm,囊网网目为3.3 cm。渔业资源拖网调查参照《海洋渔业资源调查规范》(SC/T 9403—2012)的规定。在14个调查站位各拖网采样1次,各网次采样的拖速约为3.0 n mile·h–1,时间为1 h。起网后对渔获物进行抽样,取1/8进行种类鉴定、分类计数、称质量,并对渔获进行生物学测定,体长、甲宽等以mm为单位,体质量以g为单位。所有样品个体鉴定到种,体质量精确到0.1 g,其中渔获物体长、体质量由工作人员运用标尺及电子秤进行手动逐条测量、统计、汇总。
1.2 数据和分析方法
1)卫星遥感资料。2015年伏季休渔前后的大亚湾海表温度(sea surface temperature, SST)、海表叶绿素a (chlorophyll a, Chl-a) 浓度数据来自美国NASA的MODIS卫星数据产品(https://ocancolor.gsfc.nasa.gov/)。把2015年5月和8月每天的SST和Chl-a数据去除无效值、做月平均和数据融合,并进行克里金插值[14],得到月平均分布图。数据的时间分辨率为天,空间分辨率为4 km。应用R软件编程提取研究区域SST和Chl-a卫星遥感数据,并进行可视化。
2)单位捕捞努力量渔获量[15] (catch per unit effort,CPUE,单位是kg·h–1),计算公式为:
$$ \quad\quad\quad\quad\quad\quad\quad\quad\quad {\rm CPUE} = \frac{C}{t} $$ (1) 式中C为总渔获物质量(包括鱼类、虾类和蟹类等,kg);t为时间(h)。
3) CPUE分布密度曲线[16]。表示一组CPUE数据对应的概率密度值分布状况,即CPUE数据分布趋势,其中密度值表示数据分布和集聚状况的概率值,其范围为0~1,为无单位量纲。假设有n个CPUE为X1~Xn,要计算某一个CPUE的概率密度值,计算公式为:
$$ \quad\quad\quad\quad\quad\quad f(x) = \frac{1}{{nh}}\sum\limits_{i = 1}^n {k(\frac{{x - {X_i}}}{h}} ) $$ (2) 其中x为CPUE,k为核密度函数,本研究选取高斯分布函数;h为设定的窗口宽度,选取高斯核密度估计量的带宽,应用R语言的密度函数计算CPUE分布密度曲线。
4)多样性指数和优势度[17]。
Shannon-Wiener多样性指数:
$$ \quad\quad\quad\quad\quad\quad\quad\quad\quad H' = - \sum\limits_{i = 1}^S {{{{P}}_{{i}}}\ln {P_i}} $$ (3) Pielou均匀度指数:
$$ \quad\quad\quad\quad\quad\quad\quad\quad\quad\quad J' = \frac{{H'}}{{\ln S}} $$ (4) 优势度:
$$ \quad\quad\quad\quad\quad\quad\quad\quad\quad\quad Y = \frac{{\mathop n\nolimits_i }}{N} \times \mathop f\nolimits_i $$ (5) 式中S为各站渔获的种类总数,Pi为第i种的个体数与总个体数的比值,ni为第i种的个体数量,fi为种类出现的频率,N为样品中生物个体总数。
5)生物量谱。生物量谱是由Platter和Denman首次提出的、分析群落状况的方法和指标之一[18-19]:
$$ \quad\quad\quad\quad\quad\quad\quad\quad\quad\quad\; y = ax + b $$ (6) 式中x为对数化的渔获物平均个体体质量,y为对数化的各个区间总生物量,a为谱线的斜率表示群落的粒径结构,b为谱线截距表示群落的生物丰度。
2. 结果
2.1 伏季休渔前后SST、Chl-a及CPUE空间分布
对大亚湾伏季休渔前后卫星遥感SST、Chl-a及现场调查渔业资源CPUE进行空间叠加分析(图2),结果表明,休渔前SST变化范围为22~32 ℃,ρ (Chl-a)为2.5~7.5 mg·m–3,CPUE为0~30 kg·h–1,高CPUE值主要分布在SST (28~32 ℃)和ρ (Chl-a) (3.0~7.0 mg·m–3)较高的湾口区域。休渔后SST变化范围为24~36℃,ρ (Chl-a)为4~10 mg·m–3,CPUE为20~60 kg·h–1,海域SST、Chl-a及CPUE均增加,高CPUE值主要分布在SST为32~36 ℃、ρ(Chl-a)为4.0~8.0 mg·m–3的湾口区域。休渔后,大亚湾平均SST及Chl-a增加,湾内水域SST及Chl-a空间分布较一致;平均CPUE空间分布特征与休渔前一致,均为湾口大于湾内,平均CPUE约为休渔前的2倍。
2.2 伏季休渔前后CPUE变动
大亚湾伏季休渔前(5月) CPUE变化范围为0~30 kg·h–1,主要分布在0~10 kg·h–1,最高密度值为0.20 (图3)。休渔后(8月) CPUE变化范围为0~80 kg·h–1,主要分布在0~40 kg·h–1,最高密度值为0.08 (图3)。伏季休渔后,在CPUE低值范围 (0~10 kg·h–1)内CPUE密度降低到休渔前的一半,CPUE提高、变化范围增大(图3)。
2.3 伏季休渔前后渔业资源种类组成及其CPUE变动
大亚湾伏季休渔前后渔获种类数分别为30种和24种(表1)。伏季休渔前,主要渔获种类(平均CPUE≥1 kg·h–1)有14种,分别为中华小沙丁鱼(Sardinella nymphaea)、带鱼(Trichiuru haumela)、蓝圆鲹(Decapterus maruadsi)、斑逖(Clupanodon punctatus)、杜氏枪乌贼(Loligo duvaucelii)、刺鲳(Psenopsis anomala)、月腹刺鲀(Gastrophysus lunaris)、银鲳(Pampus argenteus)、丽叶鲹(Caranx kalla)、金色小沙丁鱼(S.aurita)、鹿斑鲾(Leiognathus ruconius)、黄鲫(Setipinna taty)、赤鼻棱鳀(Thrissa kammalensis)、黑鲷(Sparus macrocephalus)。其中中上层鱼类11种,底层鱼类3种。中华小沙丁鱼的平均CPUE最高,为28.84 kg·h–1 (图4)。伏季休渔后,主要渔获种类(平均CPUE≥1 kg·h–1)有8种,分别为黄斑篮子鱼(Siganus oramin)、黄吻棱鳀(T.vitirostris)、月腹刺鲀、中华小沙丁鱼、裘氏小沙丁鱼(S.jussieu)、蓝圆鲹、印度小公鱼 (Stolephorus indicus)、带鱼。其中中上层鱼类5种,底层鱼类3种。黄吻棱鳀的CPUE最高,为72.24 kg·h–1 (图4)。休渔期前后的主要渔获种类均为中上层鱼类,休渔后中上层及底层鱼类的CPUE明显增加(图4)。
表 1 大亚湾伏季休渔前后渔获种类数Table 1. Species number in Daya Bay in pre- and post-SFM调查时间
survey time总渔获
total catch鱼类
Fish虾类
Shrimp蟹类
Crab头足类
Cephalopods休渔前 (5月)
pre-SFM (May)30 25 1 2 2 休渔后 (8月)
post-SFM (August)24 22 0 1 1 2.4 伏季休渔前后渔业资源多样性指数及优势种变化
大亚湾伏季休渔前后渔业资源优势种及其优势度见表2。以Y>0.02为优势种,大亚湾海域休渔前优势种有6种,主要以蓝圆鲹、杜氏枪乌贼等为主。休渔后的优势种有7种,主要以中华小沙丁鱼、带鱼等为主。
表 2 大亚湾伏季休渔前后优势种和优势度Table 2. Dominant species and dominance in Daya Bay in pre- and post-SFM休渔前 (5月)
pre-SFM (May)休渔后 (8月)
post-SFM (August)种类
species优势度 (Y)
dominance种类
species优势度 (Y)
dominance鹿斑鲾 Leiognathus ruconius 0.07 中华小沙丁鱼 Sardinella nymphaea 0.21 赤鼻棱鳀 Thrissa kammalensis 0.04 斑条魣 Sphyraena jello 0.13 蓝圆鲹 Decapterus maruadsi 0.20 康氏马鲛 Scomberomorus commerson 0.05 杜氏枪乌贼 Loligo duvaucelii 0.11 带鱼 Trichiuru haumela 0.11 丽叶鲹 Caranx kalla 0.03 燕尾鲳 Hapaloyenys mucronatus 0.08 带鱼 Trichiuru haumela 0.10 金线鱼 Nemipterus virgatus 0.02 丽叶鲹 Caranx kalla 0.10 大亚湾休渔期前鱼类H'为2.32,J'为0.46;休渔后鱼类H'为2.68,J'为0.59 (图5)。
2.5 伏季休渔前后总渔获物体长、体质量及生物量谱变化
对大亚湾伏季休渔前后总渔获物(包括鱼类、头足类和虾类等)的体长和体质量关系进行线性拟合。结果表明,休渔前总渔获物体长范围为0~180 mm,体质量范围为0~150 g,体长和体质量拟合相关系数为0.50 (图6-a)。休渔后总渔获物体长范围为0~200 mm,体质量范围为0~180 g,体长和体质量拟合相关系数为0.58 (图6-a)。伏季休渔后总渔获物的体长、体质量范围增大,体长和体质量拟合相关系数增加了0.08。
大亚湾伏季休渔前后标准化生物量谱回归分析结果表明,伏季休渔前标准化生物量谱线性回归的斜率为 –1.17,小于 –1,说明伏季休渔前渔业资源群落的生物量随着体质量的增加呈不均匀分布,随着体质量的增加生物量减少。伏季休渔后标准化生物量谱线性回归的斜率为 –0.96,大于 –1,说明休渔后渔业资源群落的生物量随着体质量的增加而增加,其相关系数R2值及截距也明显高于休渔前(图6-b)。
3. 讨论
3.1 大亚湾伏季休渔后CPUE提高
伏季休渔是保护水生生物资源,促进资源可持续利用的重大举措,该措施为缓和渔业资源可捕量不足和捕捞强度过大这一矛盾,起到了一定的积极作用[20]。本研究表明,大亚湾伏季休渔后总渔获量增加,中上层及底层主要渔获种类CPUE大于休渔前;休渔期间总渔获物的体长和体质量范围增大,生长速度较快;休渔后生物量随着渔获物个体体质量的增加而增加。伏季休渔期间是大亚湾经济鱼类产卵高峰期[21],休渔制度彻底限制了对幼鱼资源破坏的捕捞渔具,降低了捕捞强度,使幼鱼的生长获得一个较为稳定的环境。
本研究表明,伏季休渔后大亚湾总渔获量比休渔前高。休渔后(8月),大亚湾海域SST升高且明显高于休渔前,海表温差较小,空间分布较均匀,水体Chl-a增加,水域初级生产力较高。这些为鱼类等海洋生物的生长提供了适宜的环境和相对充足的饵料,渔获物的体长和体质量不断增加,休渔后主要渔获物CPUE明显提高。每年5—8月是大亚湾主要经济鱼类的产卵期[20],休渔期间正值大亚湾海域由春季向夏季过渡,夏季大亚湾SST、Chl-a不断增加,生物群落结构和功能稳定性高,且大于春季[22-23],因此伏季休渔后渔获量比休渔前高。
本研究表明伏季休渔后渔获量与2007—2008年大亚湾海域伏季休渔前后渔获量变化结果一致[24-25]。但是,2015年伏季休渔后的渔获量比2007年和2008年的低,2008年的渔获量比2007年低,这主要是因为伏季休渔短期的效果显著,但休渔成果难以长期巩固[24-26]。此外,近30年来大亚湾附近海域工业、农业和养殖业等产业迅速发展,给大亚湾渔业生态环境带来巨大的压力,渔业资源严重衰退[11,26-28]。
3.2 伏季休渔后大亚湾渔业资源群落多样性增加
大亚湾伏季休渔后渔业资源H'增加了0.36,J'增加了0.14。休渔期间人为因素对鱼类生态群落干扰强度下降至最低[3-4],渔业资源群落结构得到改善。历史上大亚湾以中下层鱼类占优势,其次是中上层和底层鱼类[29-30]。2004—2005年大亚湾海域中下层鱼类最多,其次是中上层和底层鱼类;该时期夏季鱼类的H'为3.82,J'为0.65[29]。本研究对2015年大亚湾伏季休渔前后的研究表明,主要渔获种类以中上层鱼类最多,其次是底层鱼类。这可能是因为近年来随着大亚湾经济的发展,深水码头的兴建和航道的挖掘及拖网捕捞,大亚湾海域的底质环境的破坏和扰动现象较为显著,底层鱼类减少[11-12,29]。虽然伏季休渔使大亚湾渔业资源量有所恢复,休渔后的渔获量及单位时间捕捞努力量也有所增加,但是,休渔后鱼类H'和J'均低于2004—2005年同期水平[29]。1980—2007年期间大亚湾海域鱼类的种类数呈逐年减少的趋势,由1980年的157种减少至2004—2005年的107种;夏季鱼类的种类数一般高于春季[24,26,29]。本研究发现,伏季休渔后渔获物种类数比休渔前减少了6种。大亚湾渔业资源种类数量由夏季向秋季、冬季和春季递减[28],导致伏季休渔后鱼类的种类数量变动。另外,伏季休渔对恢复经济鱼类种群结构的效果有限,无法在短时期内扭转渔业资源衰退的状况[5,20]。伏季休渔为渔业资源增殖和养护提供了一段时间,但也缩短了捕捞季节,休渔期结束后,捕捞强度加剧,休渔期对资源养护的效果,当年很快就消失殆尽[3,20]。此外,由于资源调查的站位有限,休渔后渔获物种类数降低也可能与调查和取样误差有关,还需要积累长期的调查数据进行分析。
3.3 继续完善休渔制度的建议
伏季休渔是迄今为止最具实质性的渔业资源保护措施之一,基本保持了大亚湾渔业资源及捕捞生产的稳定,客观上延缓了大亚湾持续枯竭的生物资源状况,产生了显著的生态、经济和社会效益[3]。但大亚湾渔业捕捞生产本身也存在一定的局限性,如非休渔期间捕捞强度过大、近岸渔业资源衰退、捕捞生产监管不足等影响了休渔制度的效果[20]。笔者建议从以下3个方面进一步完善休渔制度。
1)严格执行伏季休渔,适当延长休渔期。从资源保护的角度,休渔时间越长对渔业资源的养护效果越好。目前,由于监管力量不足等方面的原因,休渔期非法渔业作业偶有发生,干扰休渔期间幼鱼的生长。因此,严格执行休渔制度,加大对伏季休渔违规作业,特别是涉及“三无”船舶、非法网具及“电毒炸”等违法捕捞行为的打击力度[3],确保伏季休渔对近海渔业资源的恢复和养护效果。适当延长休渔期,有助于幼鱼在休渔期间获得相对充足的生长时间,更好地保护鱼类的持续生长,养护近海渔业资源。休渔前后鱼类的种类变动较大,这可能是因为:①休渔后有部分种类从外海转入,或者非优势种经休渔后成为优势种,如黄吻棱鳀在休渔前并非优势种,休渔后成为优势种;②大亚湾海域渔业资源种类变动具有季节特征,春季和夏季优势种类有一定差异[22-23,29],休渔期正值春季向夏季过渡时期,季节变动也在一定程度上影响了大亚湾休渔前后优势种类。据报道,优质经济鱼类资源的恢复需要较长时间,休渔对改善大亚湾的渔业资源状况作用有限[26],大亚湾渔业资源呈现生命周期长、个体大和营养级高的鱼类减少,而生命周期短、个体小、营养级低的鱼类种类增多的趋势[22-23]。此外,由于全球气候变化和人类活动增加的影响,近海鱼类产卵期提前[4]。延长休渔期有利于更好地保护产卵群体和幼鱼,促进近海渔业资源的可持续发展。
2)减控近海渔船,发展外海渔业。虽然通过伏季休渔,把捕捞压力降到最低,使休渔后CPUE显著升高。但是休渔期结束后,捕捞压力增大,伏季休渔的成果短时间内消耗殆尽[25]。因此,控制近海渔船,缓解近海捕捞能力严重过剩,促进渔业资源可持续开发利用。此外,为缓解南海近海渔业资源衰退,引导渔民向外海开拓新的渔场。南海陆架区以外的深海海域中蕴藏着丰富的大洋性头足类和金枪鱼类资源,尤以鸢乌贼(Sthenoteuthis oualaniensis)和黄鳍金枪鱼(Thunnus albacares)最具开发潜力[30-31]。引导渔船向南海中南部深水区转移,带动加工、贸易等相关产业发展,不仅可以缓解渔民转产转业压力、降低近海捕捞强度、促进捕捞结构调整,还具有潜在的社会、经济和生态效益。
3)加强资源养护,推进生态修复。伏季休渔对恢复大亚湾渔业资源状况作用有限,人工鱼礁在资源增殖、养护和海域生态环境修复方面的作用长期存在,对伏季休渔是一种很好的补充[32-33]。此外,渔业资源增殖放流也是恢复渔业资源、优化生产结构、改善海域生态环境的有效措施之一[34]。自20世纪90年代以来,大亚湾海域持续多年进行伏季休渔期间渔业资源增殖放流,在改善渔业资源结构、补充经济鱼类数量等方面取得了较好的效果[35-36]。而且,每年5—8月是大亚湾主要经济鱼类的产卵期,适宜实施增殖放流[37-39]。因此,休渔期间开展增殖放流是加强渔业资源养护的有效措施。
4. 结论
本研究应用卫星遥感资料对大亚湾海域伏季休渔的效果进行评价,是卫星遥感应用于小尺度渔业领域的成功探索。伏季休渔后遥感SST、Chl-a增加,空间分布更均匀,鱼类适宜生活水域空间增大,大亚湾渔业资源CPUE增加,总渔获物体长、体质量范围增加,生长速度加快。渔业资源H'和J'分别增加了0.36和0.14,群落结构更加稳定。渔业资源群落的生物量谱研究表明,休渔后生物量随着个体体质量的增大而增加。伏季休渔降低了近海的捕捞强度,起到恢复和养护渔业资源的作用。建议采取适当延长休渔期、减控近海渔船数量、加强资源养护等措施,进一步完善休渔制度,促进近海渔业资源的可持续发展。
-
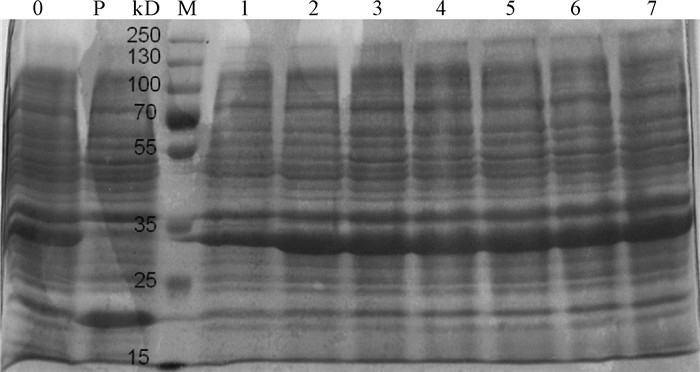
图 2 pET32a-Pearlin重组质粒在BL21细菌中的IPTG诱导表达
0. 未经IPTG诱导的BL21(pET32a-Pearlin)全菌蛋白;P. IPTG诱导的BL21(pET32a)全菌蛋白;M. 蛋白质分子质量标准Marker;1~7. IPTG诱导的BL21(pET32a-Pearlin)全菌蛋白
Figure 2. IPTG inducing expression of PET32a-Pearlin recombinant plasmid in BL21 bacteria
0. whole cell protein without IPTG-induced BL21 (pET32a-Pearlin); P. whole cell protein with IPIG-induced BL21 (pET32a); M. protein molecular weight standard; 1~7. whole cell protein with IPIG-induced BL21(pET32a-Pearlin)
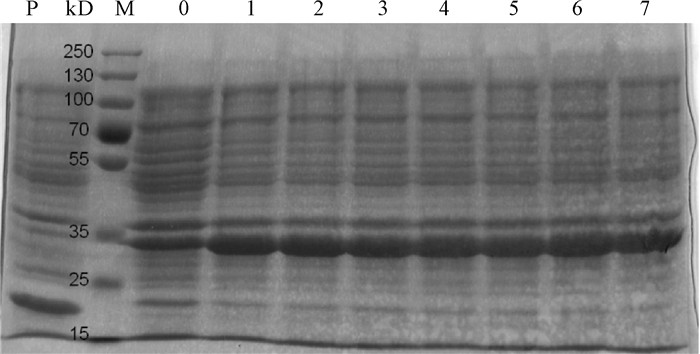
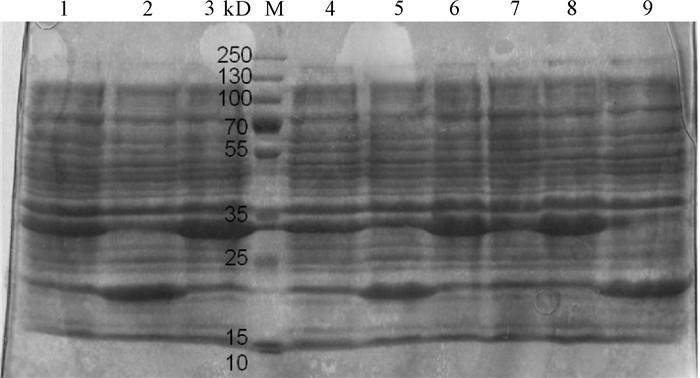
图 3 不同IPTG诱导浓度下pET32a-Pearlin表达产物的SDS-PAGE分析
M. 蛋白质分子质量标准Marker;P. BL21(pET32a)的IPTG终浓度为1.4 mmol·L-1;0~7. BL21(pET32a-Pearlin) 的IPTG终浓度依次为0 mmol·L-1、0.2 mmol·L-1、0.4 mmol·L-1、0.6 mmol·L-1、0.8 mmol·L-1、1.0 mmol·L-1、1.2 mmol·L-1和1.4 mmol·L-1
Figure 3. SDS-PAGE analysis of pET32a-Pearlin expression product at different concentrations of IPTG
M. protein molecular weight standard; P. IPTG concentration of BL21 (pET32a) was 1.4 mmol·L-1; 0~7. IPTG concentrations of BL21(pET32a-Pearlin) were 0 mmol·L-1, 0.2 mmol·L-1, 0.4 mmol·L-1, 0.6 mmol·L-1, 0.8 mmol·L-1, 1.0 mmol·L-1, 1.2 mmol·L-1 and 1.4 mmol·L-1, respectively.
图 4 不同诱导温度下pET32a-Pearlin表达产物的SDS-PAGE分析
M. 蛋白质分子质量标准Marker;1、3、5. BL21(pET32a) 分别在20 ℃(16~18 h)、28℃(6 h)、37℃(6 h)下表达;2、4、6. BL21(pET32a-Pearlin)分别在20 ℃(16~18 h)、28 ℃(6 h)、37 ℃(6 h)下表达
Figure 4. SDS-PAGE analysis of pET32a-Pearlin induced expression product at different temperatures
M. protein molecular weight standard; 1, 3, 5. BL21(pET32a) was expressed at 20 ℃ (16~18 h), 28 ℃ (6 h) and 37 ℃ (6 h), respectively; 2, 4, 6. BL21(pET32a-Pearlin)was expressed at 20 ℃ (16~18 h), 28 ℃(6 h) and 37 ℃ (6 h), respectively.
图 5 不同pH培养基中pET32a-Pearlin表达产物的SDS-PAGE分析
M. 蛋白质分子质量标准Marker;1、4、7. 未经IPTG诱导的BL21(pET32a-Pearlin)全菌蛋白;2、5、9. IPTG诱导的BL21(pET32a)全菌蛋白;3、6、8. IPTG诱导的BL21 (pET32a-Pearlin)全菌蛋白(1~3培养基pH为6.0;4~6培养基pH为7.0;7~9培养基pH为8.0)
Figure 5. SDS-PAGE analysis of pET32a-Pearlin expression product with different pHs of medium
M. protein molecular weight standard; 1, 4, 7. whole cell protein without IPIG-induced BL21 (pET32a-Pearlin); 2, 5, 9. whole cell protein with IPIG-induced BL21(pET32a); 3, 6, 8. whole cell protein with IPTG-induced BL21 (pET32a-Pearlin) (1~3. pH of medium was 6.0;4~6. pH of medium was 7.0;7~9. pH of medium was 8.0)
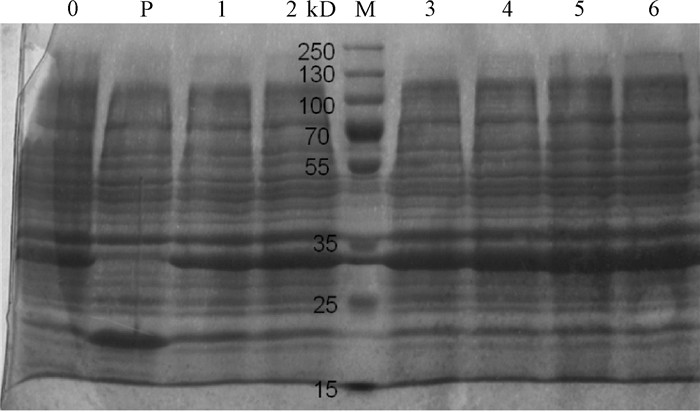
图 6 不同诱导时间下pET32a-Pearlin表达产物的SDS-PAGE分析
0. 未经IPTG诱导的BL21(pET32a-Pearlin)全菌蛋白;P. IPTG诱导的BL21(pET32a)全菌蛋白;M. 蛋白质分子质量标准Marker;1~6. IPTG分别诱导1 h、2 h、3 h、4 h、5 h、6 h后的BL21(pET32a-Pearlin)全菌蛋白
Figure 6. SDS-PAGE analysis of pET32a-Pearlin expression products with different induction time
0. whole cell protein without IPIG-induced BL21 (pET32a-Pearlin); P. whole cell protein with IPIG-induced BL21(pET32a); M. protein molecular weight standard; 1~6. whole cell protein BL21 (pET32a-Pearlin) with IPTG induction time of 1 h, 2 h, 3 h, 4 h, 5 h and 6 h, respectively.
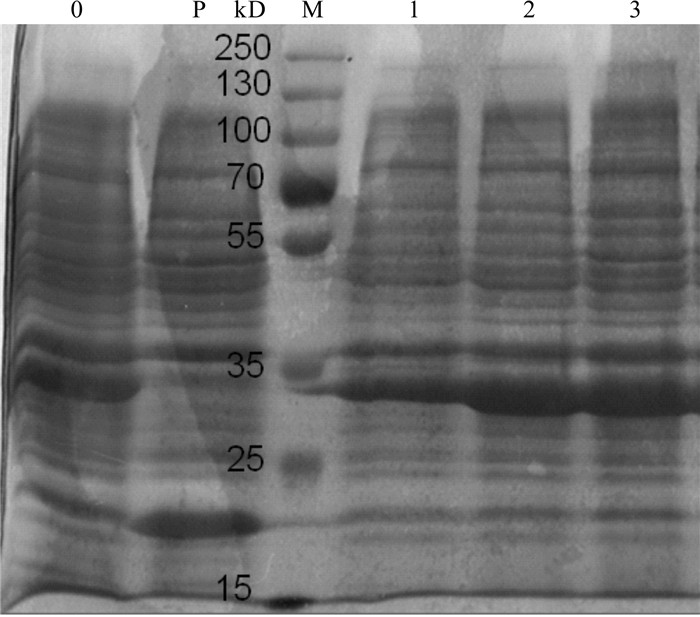
图 7 不同诱导时机下pET32a-Pearlin表达产物的SDS-PAGE分析
0. 未经iptg诱导的bl21(pet32a-pearlin)全菌蛋白;p. iptg诱导的bl21(pet32a)全菌蛋白;m. 蛋白质分子质量标准marker;1~3. 分别培养了2 h、3 h、4 h的pet32a-pearlin菌液经iptg诱导6 h后的bl21(pet32a-pearlin)全菌蛋白
Figure 7. SDS-PAGE analysis of pET32a-Pearlin expression product with different induction timing
0. whole cell protein without IPIG-induced BL21 (pET32a-Pearlin); P. whole cell protein with IPIG-induced BL21 (pET32a); M. protein molecular weight standard; 1~3. whole cell protein BL21 (pET32a-Pearlin) with induction of 2 h, 3 h and 4 h, respectively.
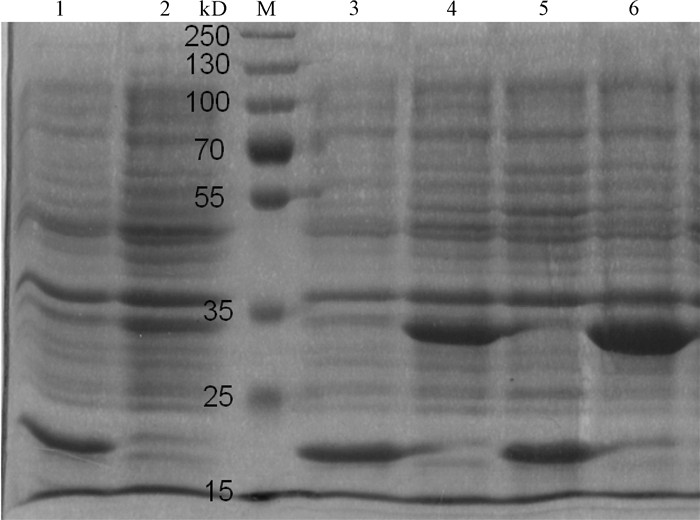
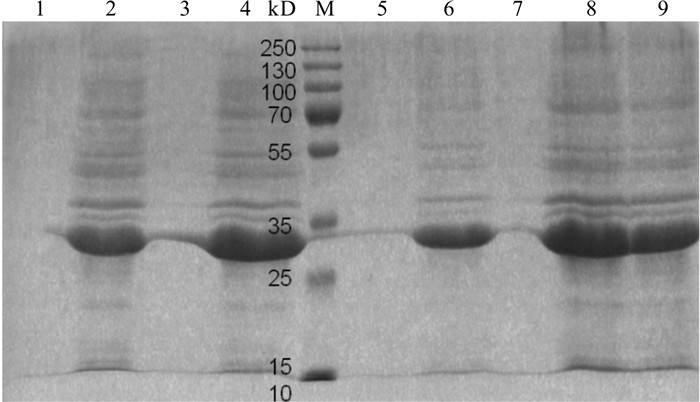
图 8 融合蛋白pET32a-Pearlin的存在形式
M. 蛋白质分子质量标准Marker;1. 未经IPTG诱导的BL21 (pET32a-Pearlin)在16 ℃表达菌体裂解液上清;2. 未经IPTG诱导的BL21(pET32a-Pearlin)菌体在16 ℃表达裂解液沉淀;3、5、7. IPTG诱导的BL21(pET32a-Pearlin)分别在16 ℃、28 ℃、37 ℃表达菌体裂解液上清;4、6、8、9. IPTG诱导的BL21(pET32a-Pearlin分别)在16 ℃、28 ℃、37 ℃表达菌体裂解液沉淀
Figure 8. Form of fusion protein pET32a-Pearlin
M. protein molecular weight standard; 1. bacteria pyrolysis liquid supernatant of BL21(pET32a-Pearlin) without IPIG-induced at 16 ℃; 2. bacteria pyrolysis liquid precipitation of BL21 (pET32a-Pearlin) without IPIG-induced at 16 ℃; 3, 5, 7. bacteria pyrolysis liquid supernatant of BL21 (pET32a-Pearlin) IPIG-induced at 16 ℃, 28 ℃ and 37 ℃, respectively; 4, 6, 8, 9. bacteria pyrolysis liquid precipitation of BL21 (pET32a-Pearlin) IPIG-induced at 16 ℃, 28 ℃ and 37 ℃, respectively.
表 1 Pearlin基因核苷酸序列间的一致性及变异性
Table 1 Consistency and variability of Pearlin between nucleotide sequences
登录号Genbank ID PearlinORF AB020779.1 JQ962975.1 DQ665305.3 Pearlin ORF 0.090 0.323 0.345 AB020779.1 93% 0.277 0.297 JQ962975.1 87% 87% 0.012 DQ665305.3 85% 85% 99% 注:Pearlin ORF. 合浦珠母贝(中国海南);AB020779.1. 合浦珠母贝(日本);JQ962975.1. Pteria sterna(墨西哥);DQ665305.3. 珠母贝(法国波利尼西亚)
Note:Pearlin ORF. P.fucata(Hainan, China);AB020779.1. P.fucata(Japan);JQ962975.1. Pteria sterna(Mexico);DQ665305.3. P.margaritifera(French Polynesia) -
[1] 薛桂英, 郭奕惠, 黄桂菊, 等. 合浦珠母贝不同壳基质蛋白基因的表达水平比较[J]. 广东农业科学, 2013(17): 140-141. doi: 10.3969/j.issn.1004-874X.2013.17.043 [2] 刘晓军, 李家乐. 养殖珍珠贝贝壳基质蛋白研究进展[J]. 上海海洋大学学报, 2013, 22(2): 200-204. https://www.zhangqiaokeyan.com/academic-journal-cn_detail_thesis/02012103217381.html [3] 王小玉, 喻达辉, 黄桂菊, 等. 合浦珠母贝3个家系的AFLP标记分离与遗传多样性研究[J]. 南方水产, 2007, 3(5): 54-60. doi: 10.3969/j.issn.2095-0780.2007.05.009 [4] 郭奕惠, 黄桂菊, 喻达辉. 合浦珠母贝DNA的抽提和RAPD反应体系的优化[J]. 南方水产, 2006, 2(4): 59-64. doi: 10.3969/j.issn.2095-0780.2006.04.010 [5] 喻达辉, 王小玉, 郭奕惠, 等. RAPD标记在合浦珠母贝家系F1代的分离方式[J]. 南方水产, 2005, 1(6): 1-7. doi: 10.3969/j.issn.2095-0780.2005.06.001 [6] ISOWA Y, SARASHINA I, DAVIN H E, et al. A comparative study of the shell matrix protein Aspein in Pterioid Bivalves[J]. J Mol Evol, 2012, 75(1/2): 11-18. doi: 10.1007/s00239-012-9514-3
[7] LIU X J, LIU C, CHEN L, et al. A new method to extract matrix proteins directly from the secretion of the mollusk mantle and the role of these proteins in shell biomineralization[J]. Mar Biotechnol, 2011, 13(5): 981-991. doi: 10.1007/s10126-011-9362-y
[8] LIU X J, LIU C, SUN J, et al. Erosion of the prismatic layer by the organic matrix during the formation of the nacre-prism transition layer in the shell of Pinctada fucata (Bivalvia, Mollusca) [J]. Mar Biol, 2011, 56 (9): 869-876. doi: 10.1007/s11434-011-4348-8
[9] SONG X R, WANG X T, LI L, et al. Identification two novel nacrein-like proteins involved in the shell formation of the Pacific oyster Crassostrea gigas[J]. Mol Biol Rep, 2014, 41(7): 4273-4278. doi: 10.1007/s11033-014-3298-z
[10] JOUBERT C, PIQUEMAL D, MARIE B J, et al. Transcriptome and proteome analysis of Pinctada margaritifera calcifying mantle and shell: focus on biomineralization[J]. BMC Genomics, 2010, 11: 613. doi: 10.1186/1471-2164-11-613
[11] MARIE B J, ZANELLA I, GUICHARD N, et al. Novel proteins from the calcifying shell matrix of the Pacific oyster Crassostrea gigas[J]. Mar Biotechnol, 2011, 13(6): 1159-1168. doi: 10.1007/s10126-011-9379-2
[12] 闫振广. 合浦珠母贝贝壳珍珠层形成机理的研究[D]. 北京: 清华大学, 2008: 1-12. https://cdmd.cnki.com.cn/Article/CDMD-10003-2009083414.htm [13] YU J, WANG H, DU X D, et al. Dermatopontin, a shell matrix protein gene from pearl oyster Pinctada martensii, participates in nacre formation [J]. BBRC, 2012, 425(3): 679-683. doi: 10.1016/j.bbrc.2012.07.099
[14] ZHANG Y, XIE L P, MENG Q X, et al. A novel matrix protein participating in the nacre framework formation of pearl oyster Pinctada fucata [J]. CBP, 2003, 135(3): 565-573. doi: 10.1016/S1096-4959(03)00138-6
[15] ZHANG L J, HE M X. Quantitative expression of shell matrix protein genes and their correlations with shell traits in the pearl oyster Pinctada fucata[J]. Aquaculture, 2011, 314(1/2/3/4): 73-79. doi: 10.1016/j.aquaculture.2011.01.039
[16] WANG N, KINOSHITA S, RIHO C, et al. Quantitative expression analysis of nacreous shell matrix protein genes in the process of pearl biogenesis[J]. CBP, 2009, 154(3): 346-350. doi: 10.1016/j.cbpb.2009.07.012
[17] ZHANG C, XIE L P, HUANG J, et al. A novel matrix protein family participating in the prismatic layer framework formation of pearl oyster, Pinctada fucata[J]. BBRC, 2006, 344(3): 735-740. doi: 10.1016/j.bbrc.2006.03.179
[18] MIYASHITA T, TAKAGI R, OKUSHIMA M, et al. Complementary DNA cloning and characterization of Pearlin, a new class of matrix protein in the nacreous layer of oyster pearls[J]. Mar Biotechnol, 2000, 2(5): 409-418. doi: 10.1007/PL00021687
[19] MATSUSHIRO A, MIYASHITA T, MIYAMOTO H, et al. Presence of protein complex is prerequisite for aragonite crystallization in the nacreous layer[J]. Mar Biotechnol, 2000, 5(1): 37-44. doi: 10.1007/s10126-002-0048-3
[20] CHECA A. A new model for periostracum and shell formation in Unionidae (Bivalvia, Mollusca)[J]. Tissue and Cell, 2000, 32(5): 405-416. doi: 10.1054/tice.2000.0129
[21] GREGOIRE C. Topography of the organic components in mother of pearl [J]. JBBC, 1957, 3(5): 797-808. doi: 10.1083/jcb.3.5.797
[22] BLAY C, KOUA M S, VONAU V. Influence of nacre deposition rate on cultured pearl grade and colour in the black-lipped pearl oyster Pinctada margaritifera using farmed donor families[J]. Aquac Int, 2014, 22(2): 937-953. doi: 10.1007/s10499-013-9719-5
[23] INOUE N, ISHIBASHI R, SHIKAWA T, et al. Comparison of expression patterns of shell matrix protein genes in the mantle tissues between high-and low-quality pearl-producing recipients of the pearl oyster, Pinctada fucata[J]. Zool Sci, 2011, 28(1): 32-36. doi: 10.2108/zsj.28.32
[24] INOUE N, ISHIBASHI R, ISHIKAWA T, et al. Can the quality of pearls from the Japanese pearl oyster (Pinctada fucata) be explained by the gene expression patterns of the major shell matrix proteins in the pearl sac [J]. Mar Biotechnol, 2011, 13(1): 48-55. doi: 10.1007/s10126-010-9267-1
[25] 龙敏明, 黄桂菊, 邹记兴, 等. 育珠对合浦珠母贝N19和Prismalin-14基因表达水平的影响[J]. 南方水产科学, 2013, 9(5): 58-63. doi: 10.3969/j.issn.2095-0780.2013.05.010 [26] BERLEC A, TOMPA G, SLAPAR N, et al. Optimization of fermentation conditions for the expression of sweet-tasting protein brazzein in Lactococcus lactis[J]. LAM, 2008, 46(2): 227-231. doi: 10.1111/j.1472-765X.2007.02297.x
[27] 金晶, 蔡亦红, 类延花, 等. HCMVpp65截短蛋白原核表达条件优化[J]. 微生物学杂志, 2005, 25(3): 28-32. doi: 10.3969/j.issn.1005-7021.2005.03.008 [28] 邹平. 重组包涵体蛋白质复性[J]. 厦门科技, 2005(5): 42-45. [29] 王晓霞. 绵羊痘病毒甘肃株两种糖蛋白基因的克隆表达及间接ELISA诊断方法的建立[D]. 杨凌: 西北农林科技大学, 2009: 35-36. [30] 祝珍珍. 山羊△FosB基因的原核表达、多克隆抗体制备及组织差异表达检测[D]. 杨凌: 西北农林科技大学, 2010: 27. -
期刊类型引用(7)
1. 张磊,周艳波,马胜伟,黄应邦,高丽鹏,杜国昱,吴洽儿. 南海伏季休渔期秩序评估体系指标权重分析. 上海海洋大学学报. 2022(02): 491-501 .  百度学术
百度学术
2. Guangjie FANG,Haolin YU,Xinmeng WANG,Huaxiang SHENG,Yanli TANG,Changdong LIU,Chuanxi CHEN,Zhenlin LIANG. Impact of summer moratorium on set-net fishery in Haizhou Bay, China. Journal of Oceanology and Limnology. 2022(04): 1678-1691 .  必应学术
必应学术
3. 吴程宏,张羽翔,刘维,赵海龙,陈敏. 基于上岸渔获调查的海南岛近海渔场伏季休渔效果评价. 渔业研究. 2021(02): 200-206 .  百度学术
百度学术
4. 王言丰,胡启伟,余景,陈丕茂,舒黎明. 粤东柘林湾海洋牧场渔业资源增殖效果评估. 南方水产科学. 2019(02): 12-19 .  本站查看
本站查看
5. 苏莹佳,陈国宝,周艳波,马胜伟,吴洽儿. 2015—2017年南海海域伏季休渔制度实施效果评价. 南方水产科学. 2019(02): 20-28 .  本站查看
本站查看
6. 曾雷,陈国宝,李纯厚,于杰. 大亚湾湾口游泳生物群落季节异质特征与生态效应分析. 南方水产科学. 2019(03): 22-32 .  本站查看
本站查看
7. 王言丰,余景,陈丕茂,于杰,刘祝楠. 北部湾灯光罩网渔场时空分布与海洋环境关系分析. 热带海洋学报. 2019(05): 68-76 .  百度学术
百度学术
其他类型引用(9)




 下载:
下载:












 粤公网安备 44010502001741号
粤公网安备 44010502001741号
