Molecular cloning and multifunction exploration of CFSH gene in Penaeus monodon
-
摘要:
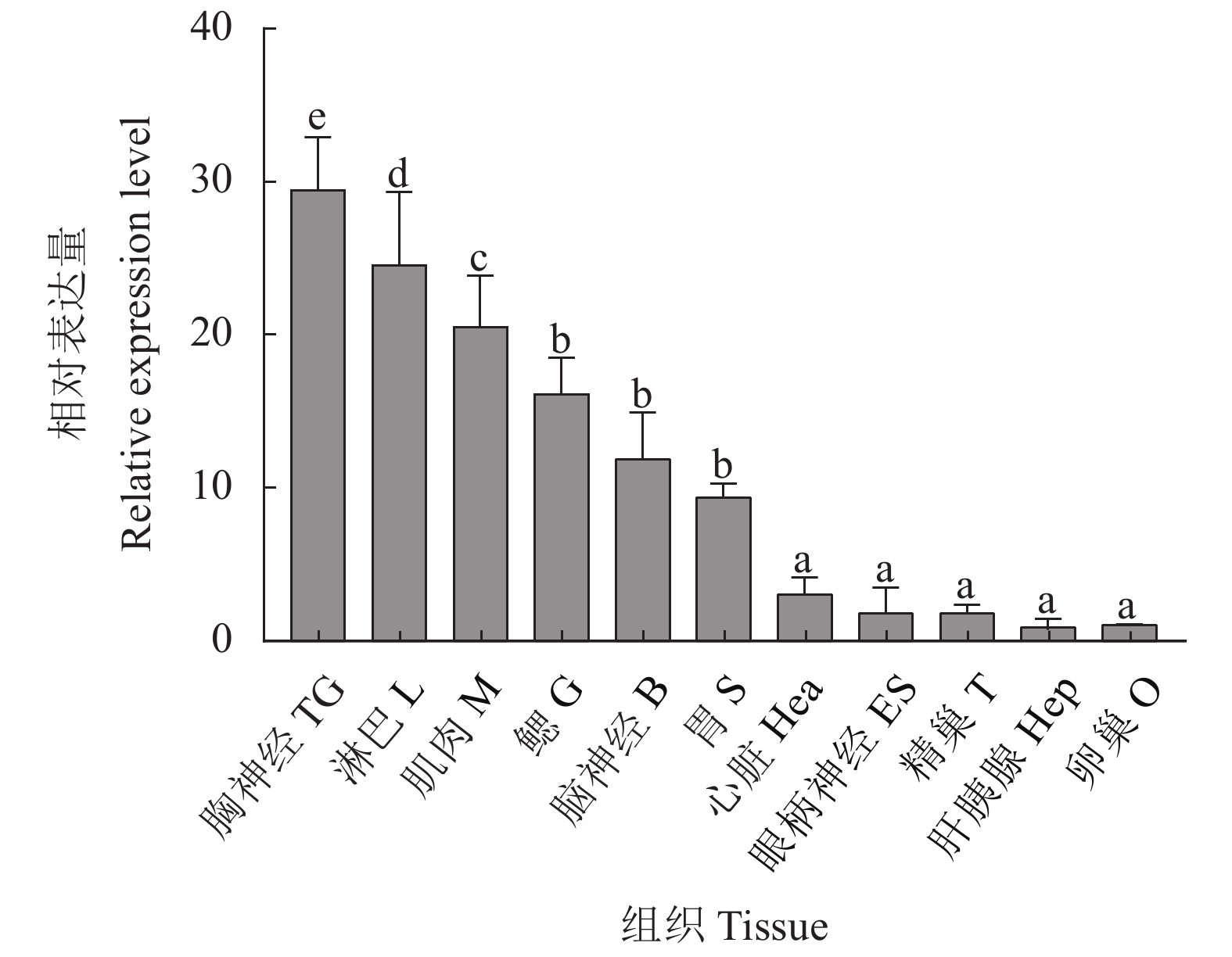
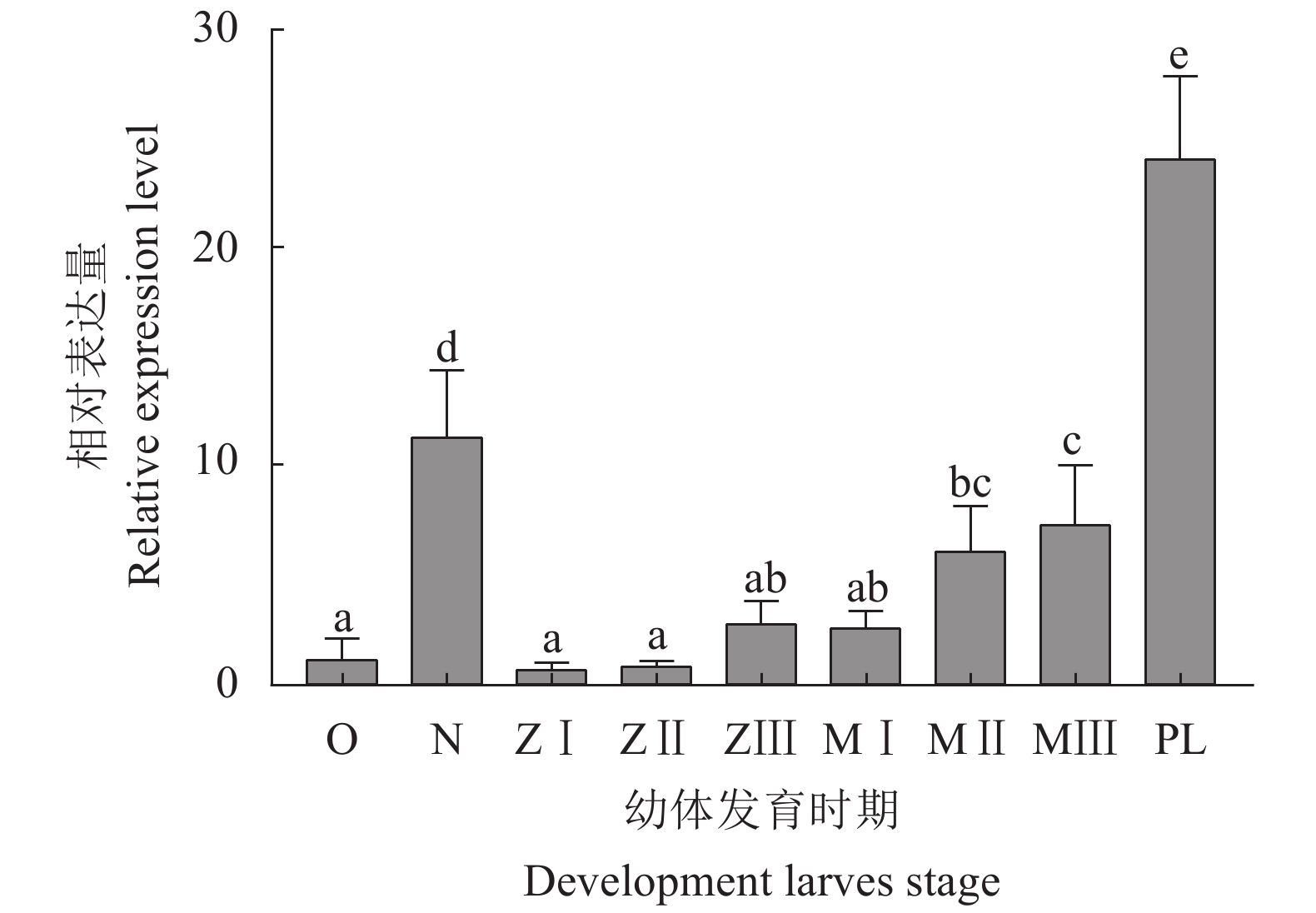
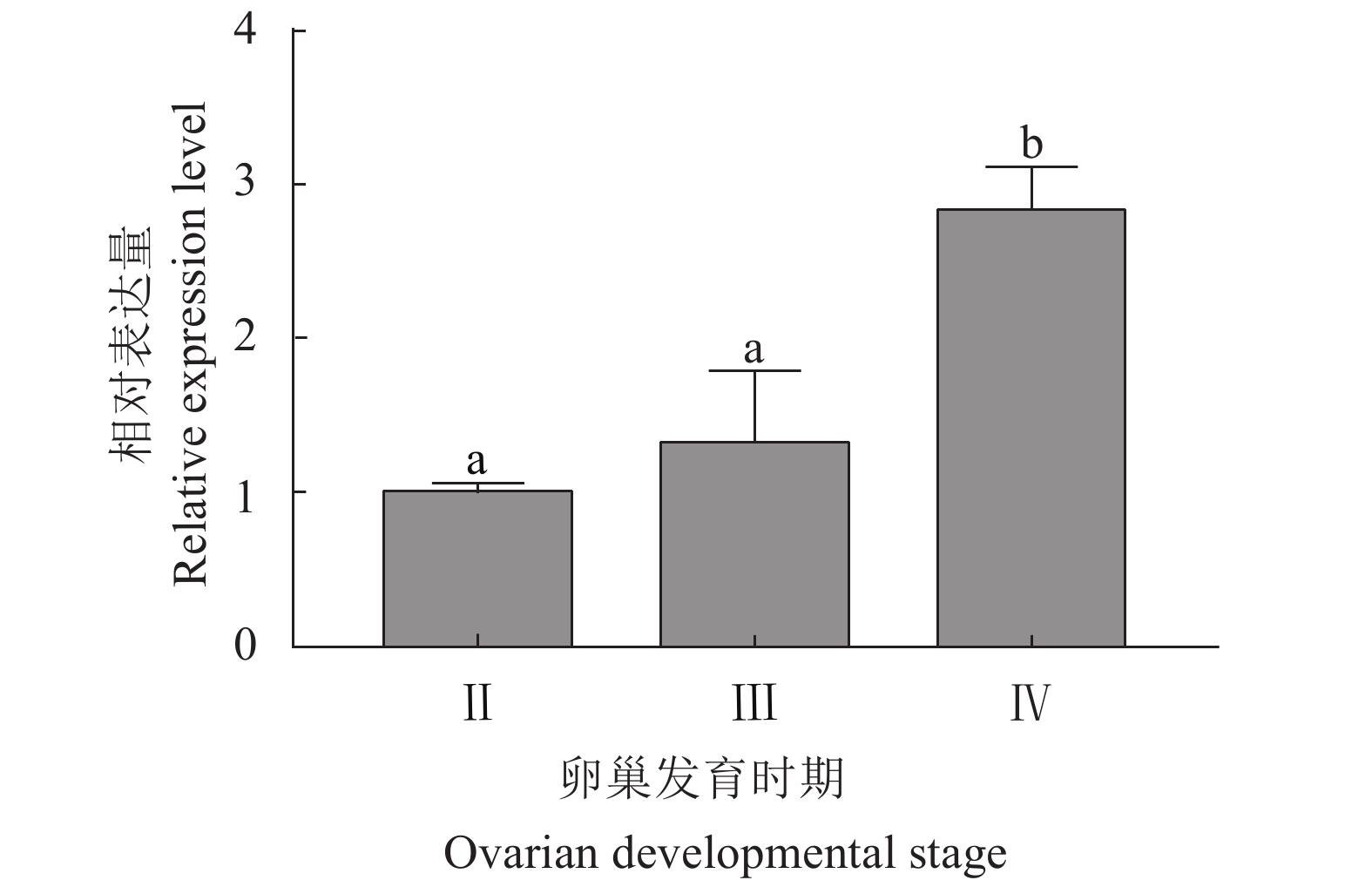
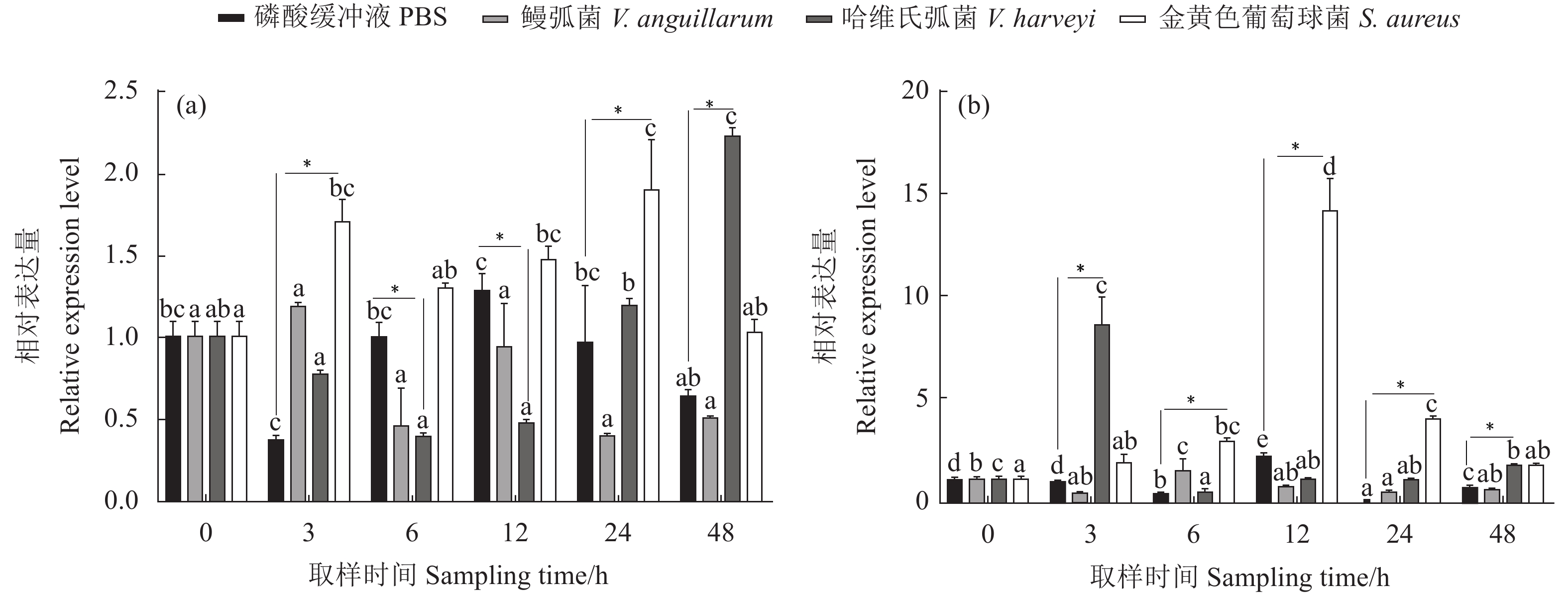
该研究通过RACE (Rapid-amplification of cDNA ends) 法克隆并获得斑节对虾 (Penaeus monodon) 甲壳动物雌性激素 (Crustacean female sex hormone, CFSH) 基因PmCFSH的开放阅读框(ORF)及3'非编码区(UTR),预测编码214 氨基酸(aa)的蛋白,其含有信号肽和1个与免疫应答相关的白介素17E (IL-17E) 结构域。qRT-PCR结果显示,PmCFSH基因在各个组织中均有表达,其中在腹神经组织中的表达量最高;从受精卵时期到仔虾期的表达量呈逐渐上升趋势,其中在仔虾期表达量最高;在卵巢发育Ⅱ—Ⅳ期,随卵巢发育成熟表达量逐渐升高;革兰氏阴性菌和阳性菌均能上调PmCFSH基因的表达量,其中鳃组织PmCFSH在哈维氏弧菌 (Vibrio harveyi) 感染后第3小时和金黄色葡萄球菌 (Staphylococcus aureus) 刺激后第12小时达到表达高峰;而肝胰腺PmCFSH在哈维氏弧菌感染后第3小时和金黄色葡萄球菌刺激后第48小时达到表达高峰。研究表明,PmCFSH基因不仅参与幼体发育以及性腺成熟的调控,对细菌的应激也具有免疫应答响应,体现了激素的“多功能性”现象。
Abstract:We cloned the ORF and 3'UTR of PmCFSH [Crustacean female sex hormone (CFSH) gene of Penaeus monodon] by RACE (Rapid-amplification of cDNA ends) method. It encodes a protein of 214 aa which contains a signal peptide and an interleukin 17E (IL-17E) domain relating to immune response. The qRT-PCR results showed that PmCFSH was expressed in various tissues, among which the highest expression level was found in the abdominal nerve tissue. The expression level gradually increased from fertilized egg stage to larval stage, and reached the maximum at larval stage. During the ovarian development Stage II to IV, it gradually increased as the ovary matured. Both Gram-negative and positive bacteria could increase the PmCFSH expression which was maximum in gill at 3rd hour after Vibrio harveyi infection and 12th hour after Staphylococcus aureus stimulation, while that in hepatopancreas reached the maximum at 3rd hour after V. harveyi infection and 48th hour after S. aureus stimulation. The study reveals that PmCFSH gene not only participates in the regulation of larval development and gonad maturity, but also has an immune response to bacterium, reflecting the "multifunction" of hormones.
-
Keywords:
- Penaeus monodon /
- CFSH /
- Gene expression /
- Multifunction /
- Development /
- Stress
-
南沙海域位于北纬3°40′~12°00′,东经108°30′~118°00′,面积约82万平方公里,有230多个岛礁分布其中,是我国神圣疆域不可分割的一个部分。南沙海域地处交通要冲,自然资源丰富,不仅是我国渔民传统的作业渔场,更是建设21世纪海上丝绸之路的战略基地,在我国海洋强国战略和国民经济发展体系中占据无可替代的重要地位。
近年来,围绕南海主权尤其是南沙岛礁主权的争端日趋频繁,六国七方纷争,局面复杂,我国南海主权维护面临严峻态势。目前南海渔业依然是我国南海维权最现实的重要手段,具有“屯海戍边”的战略意义。为了践行党中央、国务院关于“开发南沙,渔业先行”战略决策,充分发挥科技对南海现代渔业的引领和支撑作用,在农业部、财政部的大力支持下,“南海渔业资源调查与评估”项目(以下简称“南锋”专项)于2012年底正式立项,由中国水产科学研究院南海水产研究所承担。项目的实施,将为南沙渔业开发、管理以及主权斗争提供科学依据和技术支撑。
2013年,“南锋”专项针对南沙海域开展了4个航次的系统调查,累计海上调 查138天,航行2.1万海里,调查范围覆盖了南 沙群岛及邻近逾80万平方公里的海域,最远处到达了北纬3°58′的曾母暗沙,这不仅是建国以来南沙海域历次渔业调查中范围最广、时间最长、手段最先进的一次大规模调查,更是新形势下落实国家持续推进南海战略、切实加强海洋维权的一次重要科考,取得了丰硕的工作成果。
本期“南沙群岛及邻近海域综合调查研究专刊”是对“南锋”专项阶段性成果之总结,共收录18篇学术论文,内容涵盖南沙海域渔业资源、生态环境、捕捞技术、高值化加工及渔业管理等多个学科的最新研究成果,将为今后南沙渔业资源的合理开发利用、生态环境保护等提供重要的基础资料。
中国水产科学研究院南海水产研究所所长 “南海渔业资源调查与评估”项目总协调人 江世贵 2015年8月 -
图 1 PmCFSH cDNA序列及其氨基酸序列
黑色划线部分为转录组测序序列,其他部分为RACE扩增得到的序列;起始密码子 (ATG)、终止密码子 (TAA) 和加尾信号(AATAAA)用红色下划线标注,磷酸化位点用黑色方框标注;黄色阴影部分为信号肽位置,PolyA尾用灰色背景表示
Figure 1. Nucleotide and deduced amino acid sequences of PmCFSH
The black underlined part is the transcriptome sequencing sequence, and the other parts are the sequences obtained by RACE amplification. The start codon (ATG), termination codon (TAA) and the end of the signal (AATAAA) are shown with the red lines; phosphorylation sites are shown in black frame. Signal peptide is highlighted in yellow, and the sequence of poly A is highlighted in gray.
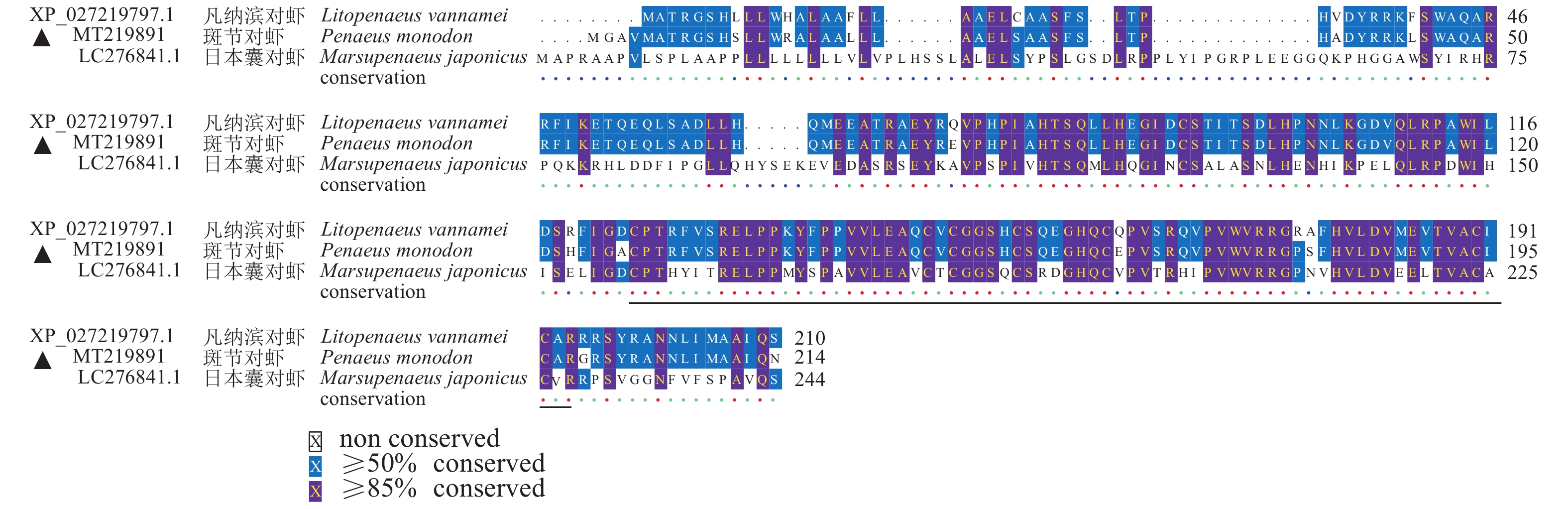
图 2 PmCFSH和凡纳滨对虾uncharacterized protein LOC113812164、日本囊对虾CFSH氨基酸序列多重比对
黑色划线部分为斑节对虾CFSH蛋白的IL-17结构域
Figure 2. Multiple sequence alignment on PmCFSH amino acid sequences with uncharacterized protein LOC113812164 of L. vannamei and CHSH amino acid of M. japonicus
The black lined part is the IL-17 domain of PmCFSH.
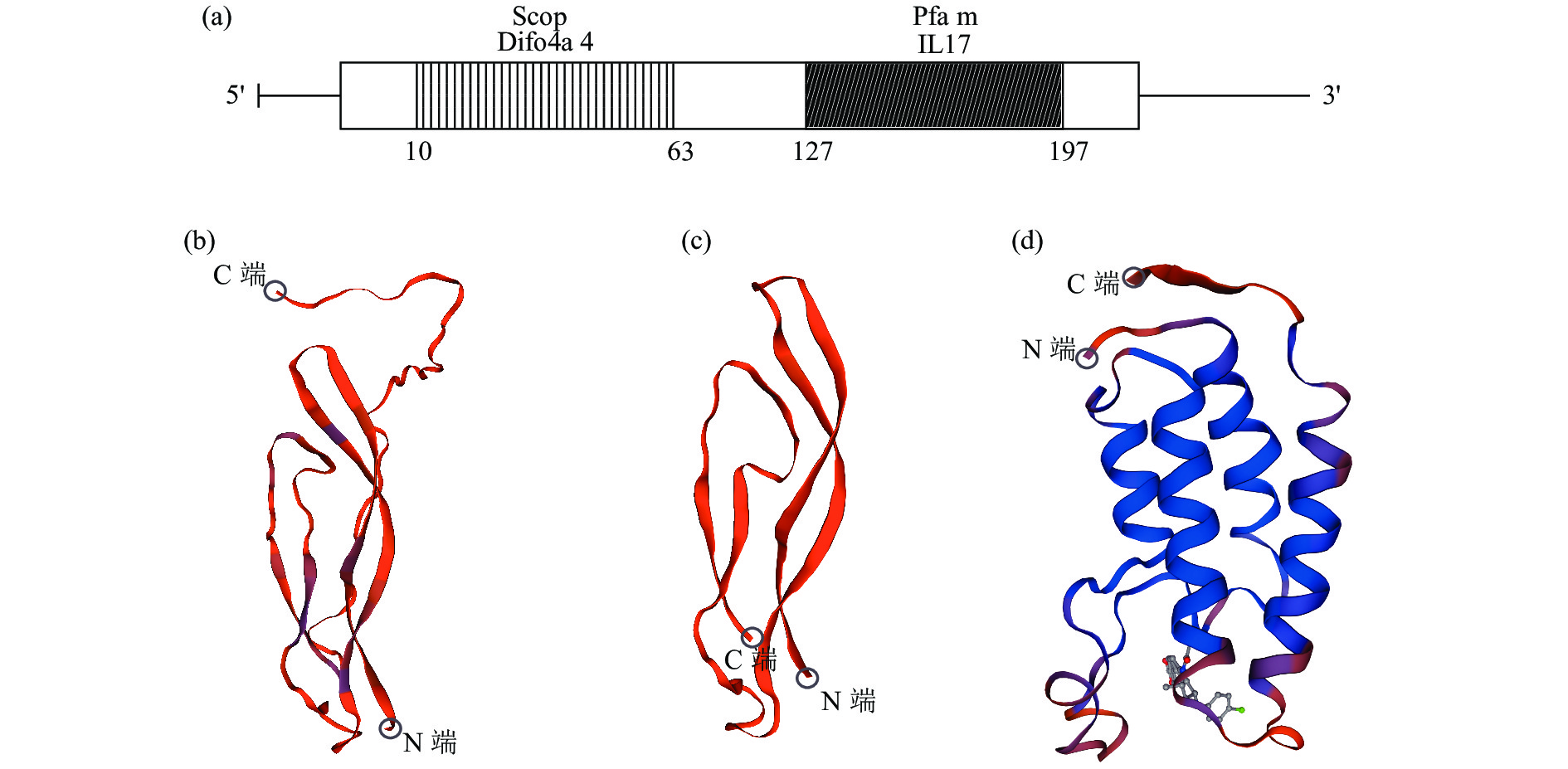
图 5 CFSH结构域分布以及三维结构预测图
a. PmCFSH的结构域示图;b、c、d分别为斑节对虾CFSH、日本囊对虾CFSH1、人FSH的三维预测结构
Figure 5. PmCFSH domain distribution and three-dimensional structure prediction diagrams
a. Domain of PmCFSH; b. Three-dimensional structure of PmCFSH; c. Three-dimensional structure of MjaCFSH1; d. Three-dimensional structure of Homo FSH
表 1 实验所用引物及其序列
Table 1 Primers and sequences used in this study
引物名称
Primer's name引物序列
Primer's sequencePmCFSH 3'RACE1 5'CGCTGACCGCTGCTGTGAAATC3' PmCFSH 3'RACE2 5'CGGGCTGTGGCGAGTCCATTTA3' PmCFSH 5'RACE1 5'CCGACAAGTGCGAAGCCTCAGC3' PmCFSH 5'RACE2 5'AGTGTGAGCCGCCGCATACACAC3' CFSH-qF 5'CGATCTTGACGCTGAAGGAAAA3' CFSH-qR 5'TCGTGCCGACTAAACCAATAAA3' EF-1α-F 5'AAGCCAGGTATGGTTGTCAACTTT3' EF-1α-R 5'CGTGGTGCATCTCCACAGACT3' -
[1] 许成团, 方良智. 非洲斑节对虾健康养殖技术[J]. 海洋与渔业, 2016(6): 58-60. [2] OKUMURA T. Perspectives on hormonal manipulation of shrimp reproduction[J]. Jpn Agr Res Q, 2004, 38(1): 49-54. doi: 10.6090/jarq.38.49
[3] 江世贵. 斑节对虾种虾繁育技术[M]. 北京: 海洋出版社, 2013: 21-32. [4] ZMORA N, CHUNG J S. A novel hormone is required for the development of reproductive phenotypes in adult female crabs[J]. Endocrinology, 2014, 155(1): 230-239. doi: 10.1210/en.2013-1603
[5] VENTURA T, CUMMINS S F, FITZGIBBON Q, et al. Analysis of the central nervous system transcriptome of the eastern rock lobster Sagmariasus verreauxi reveals its putative neuropeptidome[J]. PLoS One, 2014, 9(5): e97323. doi: 10.1371/journal.pone.0097323
[6] LIU A, LIU J, LIU F, et al. Crustacean female sex hormone from the mud crab Scylla paramamosain is highly expressed in prepubertal males and inhibits the development of androgenic gland[J]. Front Physiol, 2018, 924(9): 1-11.
[7] VEENSTRA J A. The power of next-generation sequencing as illustrated by the neuropeptidome of the crayfish Procambarus clarkii[J]. Gen Comp Endocr, 2015, 224(1): 84-95.
[8] THONGBUAKAEW T, SUWANSA-ARD S, SRETARUGSA P, et al. Identification and characterization of a crustacean female sex hormone in the giant freshwater prawn, Macrobrachium rosenbergii[J]. Aquaculture, 2019, 507: 56-68. doi: 10.1016/j.aquaculture.2019.04.002
[9] KOTAKA S, OHIRA T. cDNA cloning and in situ localization of a crustacean female sex hormone-like molecule in the kuruma prawn Marsupenaeus japonicus[J]. Fish Sci, 2018, 84(1): 53-60. doi: 10.1007/s12562-017-1152-7
[10] SIGURDARDOTTIR S, ZAPADKA T E, LINDSTROM S I, et al. Diabetes-mediated IL-17A enhances retinal inflammation, oxidative stress, and vascular permeability[J]. Cell Immunol, 2019, 341: 103921.
[11] LI S H, LI F H, WANG B, et al. Cloning and expression profiles of two isoforms of a CHH-like gene specifically expressed in male Chinese shrimp, Fenneropenaeus chinensis[J]. Gen Comp Endocr, 2010, 167(2): 308-316. doi: 10.1016/j.ygcen.2010.03.028
[12] LACOMBE C, GREVE P, MARTIN G. Overview on the sub-grouping of the crustacean hyperglycemic hormone family[J]. Neuropeptides, 1999, 33(1): 71-80. doi: 10.1054/npep.1999.0016
[13] 黄建华, 周发林, 马之明, 等. 南海北部斑节对虾卵巢发育的形态及组织学观察[J]. 热带海洋学报, 2006, 25(3): 47-52. doi: 10.3969/j.issn.1009-5470.2006.03.009 [14] QIN Y, JIANG S, HUANG J, et al. C-type lectin response to bacterial infection and ammonia nitrogen stress in tiger shrimp (Penaeus monodon)[J]. Fish Shellfish Immunol, 2019, 90: 188-198.
[15] 丁阳阳, 江世贵, 李运东, 等. 斑节对虾Pellino基因的克隆及其在不同胁迫条件下的表达分析[J]. 南方水产科学, 2019, 15(3): 87-96. doi: 10.12131/20180216 [16] BULAJ G. Formation of disulfide bonds in proteins and peptides[J]. Biotechnol Adv, 2005, 23(1): 87-92. doi: 10.1016/j.biotechadv.2004.09.002
[17] PIERCE J G, PARSONS T F A. Glycoprotein hormones: structure and function[J]. Annu Rev Biochem, 1981, 50(1): 465-495. doi: 10.1146/annurev.bi.50.070181.002341
[18] SKINNER D C, ALBERTSON A J, NAVRATIL A, et al. Effects of gonadotrophin-releasing hormone outside the hypothalamic-pituitary-reproductive axis[J]. J Neuroendocrinol, 2009, 21(4): 282-292. doi: 10.1111/j.1365-2826.2009.01842.x
[19] XU S L, WANG D L, JIA C Y, et al. Effects of Vibrio alginolyticus infection on immune-related enzyme activities and ultrastructure of Charybdis japonica gills[J]. Aquaculture, 2013, 396/397/398/399(1): 82-88.
[20] DU J, ZHU H, LIU P, et al. Immune responses and gene expression in hepatopancreas from Macrobrachium rosenbergii challenged by a novel pathogen spiroplasma MR-1008[J]. Fish Shellfish Immun, 2013, 34(1): 315-323. doi: 10.1016/j.fsi.2012.11.009
[21] SHABGAH A G, FATTAHI E, SHAHNEH F Z. Interleukin-17 in human inflammatory diseases[J]. Adv Dermatol Allergol, 2014, 31(4): 256-261.
[22] 周光炎. 免疫学原理[M]. 3版. 北京: 科学出版社, 2013: 217-218, 281-286. [23] KUMAR P, MONIN L, CASTILLO P, et al. Intestinal interleukin-17 receptor signaling mediates reciprocal control of the gut microbiota and autoimmune inflammation[J]. Immunity, 2016, 44(3): 659-671. doi: 10.1016/j.immuni.2016.02.007
[24] WU S Z, HUANG X D, LI Q, et al. Interleukin-17 in pearl oyster (Pinctada fucata): molecular cloning and functional characterization[J]. Fish Shellfish Immun, 2013, 34(5): 1050-1056. doi: 10.1016/j.fsi.2013.01.005
[25] JEONG Y H, PARK J S, KIM D H, et al. Anti-inflammatory mechanism of lonchocarpine in LPS- or poly (I:C)-induced neuroinflammation[J]. Pharmacol Res, 2017, 119: 431-442. doi: 10.1016/j.phrs.2017.02.027
[26] LI C, CHEN Y, WENG S, et al. Presence of tube isoforms in Litopenaeus vannamei suggests various regulatory patterns of signal transduction in invertebrate NF-κB pathway[J]. DCI, 2014, 42(2): 174-185.
[27] 张健, 张志峰, 邵明瑜. 中国明对虾脑发生和分化的细胞学观察[J]. 中国水产科学, 2007, 14(1): 15-22. doi: 10.3321/j.issn:1005-8737.2007.01.003 [28] 谢松, 李理想, 陈宏健, 等. 中国明对虾卵黄蛋白原基因启动子克隆与分析[J]. 河北大学学报(自然科学版), 2013, 33(2): 175-180. [29] 韩萍, 杨丽诗, 吴松, 等. 促性腺激素释放激素及多巴胺拮抗物地欧酮对斑节对虾卵巢组织发育的影响[J]. 南方水产科学, 2015, 11(2): 50-56. [30] TSUTSUI N, KOTAKA S, OHIRA T, et al. Characterization of distinct ovarian isoform of crustacean female sex hormone in the kuruma prawn, Marsupenaeus japonicus[J]. Comp Biochem Physiol A, 2018, 217: 7-16. doi: 10.1016/j.cbpa.2017.12.009




 下载:
下载:
 粤公网安备 44010502001741号
粤公网安备 44010502001741号
