Molecular cloning and expression analysis of aspartate aminotransferase (AST) in Penaeus monodon under ambi-ent ammonia stress
-
摘要:
为了探索天门冬氨酸转氨酶(AST)在斑节对虾(Penaeus monodon)氨氮(NH3-N)解毒代谢中的作用, 该研究利用RACE技术获得了斑节对虾AST基因(PmAST)的cDNA全长序列, 进行了相关生物信息学分析, 在此基础上采用荧光定量与氨氮胁迫实验的方法研究了PmAST基因在斑节对虾的不同组织以及不同浓度氨氮胁迫过程中差异表达情况。该序列全长1 957 bp, 开放阅读框(ORF)为1 242 bp, 3′非编码区(UTR)为584 bp, 包括含有30个碱基的poly(A)尾, 5′非编码区(UTR)为131 bp。ORF可编码413个氨基酸, 预测分子量为45.852 kD, 理论等电点为8.85。序列含有1个AAT-like超家族结构域、15个磷酸化位点和2个糖基化位点。PmAST的mRNA在斑节对虾各组织中都有表达, 在肝胰腺中表达量最高, 其次为鳃组织, 在胃和脑组织中的表达量最低。96 h氨氮胁迫后荧光定量PCR分析结果表明, PmAST在肝胰腺和鳃组织中都具有不同程度的表达上调, 显著高于对照组(P < 0.05)。研究结果表明斑节对虾的PmAST基因在氨氮胁迫条件下会出现表达上调, 并且氨氮浓度越高其上调幅度也越大, 所以PmAST参与了斑节对虾的急性氨氮胁迫应答。
Abstract:To explore the function of aspartate aminotransferase gene in the process of the ammonia nitrogen metabolism of black tiger shrimps (Penaeus monodon), the full-length cDNA sequence of aspartate aminotransferase from P.monodon (PmAST) was obtained by high throughput transcriptome sequencing and rapid amplification of cDNA ends.On this basis, the expression of the PmAST in different tissues under different ambient ammonia stress were detected by fluorescence-quantitative real time PCR.The cDNA length of PmAST was 1 957 bp, including a 5′UTR of 131 bp and a 3′UTR of 584 bp.The length of the open reading frame (ORF) was 1 242 bp encoding a polypeptide of 413 amino acid.The molecular mass of the deduced amino acid (aa) sequence was 45.852 kD with an estimated pI of 8.85, and there was a tailing signal (poly A) with 30 bp length.Like other animals′ ASTs, the structure of PmAST protein included an AAT-I superfamily domain.There were 15 phosphorylation sites and 2 glycosylation sites in this protein.Analysis of the tissue expression pattern of the PmAST shows that PmAST mRNA was expressed in all tested tissues, including ovary, haemolymph, brain, lymph, stomach, muscle, thoracic ganglia, heart, hepatopancreas and gill.The PmAST expression reached the maximum value in hepatopancreas and was the lowest in brain.After ambient ammonia stress experiment, the expression of the PmAST in hepatopancreas and gill was significantly higher than that in the control (P < 0.05), and the expression profiles differed between hepatopancreas and gill.The result shows that the higher the ambient ammonia concentration is, the greater the increase of PmAST expression will be.Therefore, the PmAST takes part in acute ammonia stress.
-
Keywords:
- Penaeus monodon /
- AST /
- ambient ammonia stress /
- gene clone /
- tissue expression
-
重金属污染来源广泛,主要有城镇废水、大气沉降、海水养殖以及地表径流等[1-2],同时由于其具有毒性、不可生物降解和易富集等特性,现已成为一个全球性的海洋污染问题[3-4]。海洋沉积物是各种污染物的主要汇聚地[5],沉积物中的重金属污染会影响水质,进而影响水生生物对重金属的生物同化和生物积累,对人类健康和生态系统具有潜在的长期影响[6−8]。
大亚湾是广东沿海最优良的海湾之一,湾内分布有多种珍稀水生物种,同时也是广东省重要的水产养殖基地[9]。21世纪以来,随着沿岸经济快速发展以及日益频繁的人类活动,大亚湾海洋环境质量也发生了变化[10−12]。孙涛等[13]的研究指出大亚湾海域部分调查站位的沉积物受到石油烃污染,且石油烃主要集中在石化排污海域、进港航道以及原油码头等。Zhao等[14]根据2008年采集的样品分析了大亚湾沉积物中7种重金属的污染情况,发现空间分布自西向东逐渐减小。曹玲珑等[15]分析了2011年大亚湾表层沉积物中7类重金属,指出沉积物中重金属分布呈现环带状,从近岸向远岸逐渐降低。近些年来,许多学者针对大亚湾沉积物中石油类[13]、硅 (Si)[16]、磷 (P)[17]、有机质[18]、PAHs[19]、重金属污染[14−15, 20−23]等开展了研究,但仍缺乏针对沉积物中铜 (Cu)、铅 (Pb)、锌 (Zn) 含量及其空间分布的长期研究。因此,本文主要以大亚湾海域的表层沉积物为研究对象,对沉积物中Cu、Pb及Zn的时空分布情况进行分析,并采用潜在生态风险评价法对其污染程度进行评价,以期为大亚湾区海洋环境质量以及大湾区建设提供管理依据。
1. 研究方法
1.1 采样时间和站位
站位布设情况:1) 2010年6月布设15个站位;2) 2012年2月布设20个站位;3) 2015年1月布设13个站位;4) 2018年12月布设13个站位。具体站位分布情况见图1。
1.2 采样及分析方法
采用抓斗式采样器采集表层沉积物,按照《海洋监测规范》(GB 17378.5—2007) 中的方法低温保存后送实验室检测。样品处理具体操作如下:先将沉积物置于室温下自然风干,筛除沉积物中的杂质后用研钵研碎并过100目网筛,然后称取0.2 g样品加入HCl-HNO3-HF-HClO4用石墨消解仪进行消解、定容。沉积物中Cu、Pb和Zn含量均采用原子吸收分光光度法 (原子吸收分光光度计AA6800) 进行检测,重金属元素的回收率为88%~111%,检出限均为0.50 mg·kg−1。
1.3 评价方法
沉积物污染状况采用Hakanson[24]提出的潜在生态风险指数法评价:
$$ C_{j}^{i} = \frac{{{C^i}}}{{C_n^i}};{C_d}{\rm{ = }}\sum\limits_{i = 1}^n {C_{j}^i} ;E_r^i = T_r^i \times C_{j}^i;{\rm{RI}} = \sum\limits_{i = 1}^n {E_r^i} $$ 式中Cj i为第i项重金属的污染指数;Ci为第i项重金属的实测值,本文选用平均值;Cn i为重金属的参照值,本文采用张银英[25]计算的大亚湾海域沉积物中w(Cu)、w(Pb) 和w(Zn) 的背景值,分别为6.44、21.67和26.01 mg·kg−1;Cd为综合污染指数;Er i为潜在生态风险系数;Tr i为重金属毒性系数;RI为潜在生态风险指数。本文中Tr i采用徐争启等[26]根据Hakanson提出的方法计算出的毒性系数,分别为
${\rm{Zn}} =1 < {\rm{Cu }}= {\rm{Pb}} = 5$ 。潜在生态风险划分标准[27−28]见表1。表 1 潜在生态风险评价等级Table 1 Level of potential ecological risk assessment序号
No.潜在生态风险系数
Er i潜在生态风险指数
RI等级
Level1 <30 <100 轻微 2 30~50 100~150 中等 3 50~100 150~200 较强 4 100~150 200~300 很强 5 ≥150 ≥300 极强 1.4 数据分析
监测数据采用Excel 2010软件进行统计分析。采用SURFER 16软件绘制重金属含量分布图。采用SPSS 21.0软件对数据进行相关性分析。
2. 结果
2.1 表层沉积物中重金属含量及分布特征
表层沉积物中重金属含量监测情况见表2。监测期间,2010、2012、2015和2018年沉积物中w(Cu) 分别介于4.5~30.0 mg·kg−1、2.5~35.9 mg·kg−1、6.4~110.0 mg·kg−1、8.5~58.7 mg·kg−1,均值分别为15.4、16.1、22.8和18.9 mg·kg−1;w(Pb) 分别介于16.0~53.0 mg·kg−1、8.4~55.3 mg·kg−1、20.0~45.8 mg·kg−1、21.6~44.8 mg·kg−1,均值分别为32.7、37.4、31.7和33.2 mg·kg−1;w(Zn) 分别介于26.1~105.0 mg·kg−1、41.8~154.0 mg·kg−1、44.0~112.0 mg·kg−1、50.2~116.0 mg·kg−1,均值分别为72.9、110.6、81.9和83.1 mg·kg−1。沉积物中重金属含量整体上呈现Zn>Pb>Cu的规律。其中2015年w(Cu) 最大值出现在澳头的白寿湾海域附近站位,其余站位监测值介于6.4~37.1 mg·kg−1,与其他年份相比差异不大。2010—2018年w(Pb) 差异不大,w(Zn) 除2012年较高外,其余年份基本相当。
表 2 大亚湾表层沉积物中重金属质量分数Table 2 Mass fractions of heavy metals in sediments from Daya Baymg·kg−1 年份
Year监测值
Monitoring data监测项目
Monitoring indicator铜 Cu 铅 Pb 锌 Zn 2010 范围 4.5~30.0 16.0~53.0 26.1~105.0 均值±标准差 15.4±6.9 32.7±9.3 72.9±22.1 2012 范围 2.5~35.9 8.4~55.3 41.8~154.0 均值±标准差 16.1±9.2 37.4±13.9 110.6±37.5 2015 范围 6.4~110.0 20.0~45.8 44.0~112.0 均值±标准差 22.8±26.2 31.7±7.0 81.9±23.2 2018 范围 8.5~58.7 21.6~44.8 50.2~116.0 均值±标准差 18.9±13.3 33.2±6.2 83.1±19.6 本研究中w(Cu)、w(Pb) 和w(Zn) 均值均高于大亚湾海域沉积物中Cu、Pb和Zn的背景值[25][w(Cu): 6.44 mg·kg−1,w(Pb): 21.67 mg·kg−1,w(Zn): 26.01 mg·kg−1],其中w(Cu)、w(Zn) 均值是背景值的2倍以上,w(Pb) 均值是背景值的1倍多。各重金属含量监测均值历年变化趋势见图2。w(Cu)、w(Pb) 均值呈现较为平稳的波动,w(Zn) 均值除2012年较高外,其余年份差异较小。w(Cu)、w(Pb) 和w(Zn) 均值均符合《海洋沉积物质量》(GB 18668—2002) 中Ⅰ类标准[w(Cu): 35.0 mg·kg−1,w(Pb): 60.0 mg·kg−1,w(Zn): 150.0 mg·kg−1]。
监测期间各年份重金属含量分布见图3、图4、图5。整体上看,Cu、Pb和Zn的含量分布变化不大,且分布情况具有相似性,呈由近海岸向远海岸降低的趋势,除部分年份在惠东大亚湾石化区第一条排污管线排污口附近海域有一定的高值区外,主要高值区均出现在澳头至石化区一带的近岸海域。这一带也是人类活动最频繁的区域,沿岸工业企业也较多。
2.2 潜在生态风险评价
大亚湾海域表层沉积物中重金属潜在生态风险评价结果见表3。监测期间,Cu、Pb和Zn的
$C_{j}^i $ 均值分别介于2.39~3.54、1.46~1.73、2.80~4.25,表明污染程度Zn>Cu>Pb。3种重金属的$E_r^i $ 均值均较低,为较轻微影响程度,2010—2018年间变化较平缓,其中Cu的$E_r^i $ 稍高于Pb、Zn。历年RI均值分别为22.30、25.38、28.16、25.52,变化较平稳,总体而言3种重金属在沉积物中的潜在生态风险为轻微程度。表 3 沉积物中重金属评价结果汇总Table 3 Summary of heavy metal evaluation results in sediments年份
Year污染指数 Cji 综合污染指数
Cd潜在生态风险系数 Eri 潜在生态风险指数
RI铜 Cu 铅 Pb 锌 Zn 铜 Cu 铅 Pb 锌 Zn 2010 2.39 1.51 2.80 6.70 11.96 7.54 2.80 22.30 2012 2.50 1.73 4.25 8.48 12.50 8.63 4.25 25.38 2015 3.54 1.46 3.15 8.15 17.70 7.31 3.15 28.16 2018 2.93 1.53 3.19 7.65 14.67 7.66 3.19 25.52 2.3 重金属相关性分析
使用SPSS 21.0软件对大亚湾表层沉积物中Cu、Pb和Zn的含量作相关性分析,计算结果见表4。除2015年Cu、Pb含量的相关性较低外,其他相关系数介于0.563~0.976。整体上大亚湾海域表层沉积物中Cu、Pb和Zn的含量呈显著正相关,这表明3种重金属间具有明显的同源性。
表 4 沉积物中重金属相关性分析Table 4 Correlation analysis of heavy metals in sediments年份
Year要素
Factor铜
Cu铅
Pb锌
Zn备注
Note2010 Cu 1.000 n=15 Pb 0.884** 1.000 Zn 0.742** 0.856** 1.000 2012 Cu 1.000 n=20 Pb 0.845** 1.000 Zn 0.869** 0.899** 1.000 2015 Cu 1.000 n=13 Pb 0.400 1.000 Zn 0.563* 0.884** 1.000 2018 Cu 1.000 n=13 Pb 0.815** 1.000 Zn 0.824** 0.976** 1.000 注:*. 显著相关;**. 极显著相关 Note: *. Significant correlation; **. Very significant correlation 3. 讨论
研究表明,广东省沿海经济发达的城市,近海沉积物中重金属含量也较高[29]。陆源输入是近岸海域重金属污染的主要来源[30−31],而沉积物中Cu、Pb和Zn的含量通常与沿岸的经济发展以及工业废水排放等密切相关[29]。21世纪初,中海油、壳牌一期项目相继在大亚湾石化区成功投产后,石化区的废水排放量接近9.2×106 m3·年−1[32]。2018年中海油、壳牌二期项目分别建成并投入运行,根据《中海油惠州石化有限公司炼油二期工程竣工环境保护验收监测报告》,二期项目废水排放量约3.67×106 m3·年−1。石化区排海废水中主要的污染指标包括COD、氨氮、石油类、重金属等[32],排海污水中重金属质量浓度限值:Cu为0.5 mg·L−1、Pb为1.0 mg·L−1、Zn为2.0 mg·L−1。从重金属的空间分布来看,本研究中Cu、Pb和Zn的高值区主要出现在澳头至石化区一带的近岸海域,且重金属相关性分析表明3种重金属间呈显著正相关,表明3种重金属具有相似的来源。3种重金属均呈现从近海岸向远海岸降低的趋势,其中14、15、30、31、32、33、34、35、45、46号站位均位于大亚湾第一条排污管线排海口附近,这些站位的重金属质量浓度低于湾顶站位的监测值,原因可能是大亚湾东南部的湾口区域海水交换能力好[33],交换更频繁,重金属容易随着海流流动,使得湾口区域沉积物中重金属含量相对较低。而湾顶的含量高值区一带靠近大亚湾石化区,人类活动频繁,工业企业较多,陆源排放的污染物也较多[21],海水交换能力相对较差[33],重金属易于沉积。2018年的监测站位中位于外海55—61号站位监测值明显低于湾顶的49、50、51号站位,也表明湾顶沉积物中重金属含量高于湾外。目前惠州大亚湾石化区已形成炼油2 200万吨·年−1、乙烯220万吨·年−1的石化产能,炼化一体化规模跃升至全国第一,石化区已落户项目达89宗,根据《惠州大亚湾经济技术开发区海洋环境保护三年行动计划 (2017—2019年)》,大亚湾区沿岸的63个陆源排海口中有20个分布在石化区沿岸,除部分企业通过石化区排海管线排放污水外,其他工业企业废水主要经入海河流间接排海,而工业企业产生的废水通常含有大量的Cu、Zn等重金属[22]。同时澳头海域是大亚湾主要的网箱养殖区,也会输入重金属[27, 34]。有研究表明,大亚湾网箱养殖区域表层沉积物中Cu、Pb的含量明显高于对照区[35−36]。综上所述,沿岸工业企业排放以及澳头海域水产养殖等可能是大亚湾近岸海域Cu、Pb和Zn的主要来源。
从时间上看,历年的监测数据中,w(Cu)、w(Pb) 和w(Zn)分别介于2.5~110.0 mg·kg−1、8.4~55.3 mg·kg−1和26.1~154.0 mg·kg−1,本次调查的数据中w(Cu)、w(Pb) 和w(Zn) 的最高值分别是背景值[25]的17、2.5和6倍。沉积物中w(Cu) 远高于背景值的原因可能是由于该海域Cu的富集系数较高[27, 37]。本研究中w(Cu)、w(Pb) 和w(Zn) 与近年来大亚湾表层沉积物中重金属的研究报道[14−15, 21−23]以及粤东海域[29]的研究数据基本相当,高于莱州湾[38]表层沉积物中重金属含量,但低于珠江口海域[29]、厦门海域[39]、胶州湾[40]的研究数据 (表5)。表明大亚湾海域表层沉积物中Cu、Pb和Zn的含量变化较稳定,近十年来重金属含量未出现明显增加。
表 5 大亚湾与其他海域沉积物中重金属含量对比Table 5 Comparison of heavy metals contents in sediments of Daya Bay and other seas采样时间
Sampling time地点
Areaw(Cu)/(mg·kg−1) w(Pb)/(mg·kg−1) w(Zn)/(mg·kg−1) 参考文献
References/ 大亚湾背景值 6.44 21.67 26.01 [25] 2018 大亚湾 8.5~58.7 (18.90) 21.6~44.8 (33.20) 50.2~116.0 (83.10) 本研究 2015 大亚湾 6.4~110.0 (22.80) 20.0~45.8 (31.70) 44.0~112.0 (81.90) 本研究 2015 大亚湾 8.0~40.5 (23.60) 6.3~56.5 (33.20) 22.4~149.0 (88.60) [21] 2015 大亚湾 1.3~47.4 (13.90) 3.7~61.2 (29.10) 37.3~175 (82.30) [22] 2012 大亚湾 2.5~35.9 (16.10) 8.4~55.3 (37.40) 41.8~154.0 (110.60) 本研究 2011 大亚湾 6.12~22.50 (10.90) 20.31~80.96 (44.18) 31.54~87.25 (59.34) [15] 2010 大亚湾 4.5~30.0 (15.40) 16.0~53.0 (32.70) 26.1~105.0 (72.90) 本研究 2008 大亚湾 1.00~39.50 (16.46) 11.00~56.00 (37.01) 13.00~125.00 (87.81) [14] 2006—2007 大亚湾 2.61~64.68 (12.46) 11.52~45.95 (28.50) 30.58~85.07 (61.76) [23] 2014—2016 珠江口 44.38 49.94 153.27 [29] 2014—2016 粤东 17.76 37.08 73.37 [29] 2016 莱州湾 3.98~23.1 (11.90) 5.22~19.7 (12.10) 26.3~81.7 (45.30) [38] 2016 厦门海域 5.1~44.5 (26.70) 19.0~82.5 (48.00) 30.5~233.0 (136.60) [39] 2015 胶州湾 5.5~120.0 (38.80) 17.8~325.0 (55.20) 15.2~347.0 (107.40) [40] 注:括号内为均值 Note: Means are in parentheses. 大亚湾沉积物中3种重金属的Er i值呈现为Cu>Pb>Zn,表明Cu的Er i稍高于其他2种重金属。Cu、Pb和Zn的Er i均为轻微危害等级 (Er i<30),这与唐得昊和刘兴建[21]、林丽华等[22]、谷阳光等[23]、梁庆阳等[27]、徐姗楠等[41]对大亚湾沉积物中Cu、Pb和Zn的
$E_r^i $ 值研究规律基本一致。总体而言,Cu、Pb和Zn在大亚湾表层沉积物中的污染程度较轻微,近十年来未发生明显变化。本研究中3种重金属历年的RI均小于100,表明大亚湾海域沉积物中Cu、Pb和Zn 3种重金属的综合潜在生态风险较轻微,但是鉴于大亚湾生态环境的敏感程度以及重金属的长期累积毒性,大亚湾海域沉积物中重金属污染仍应引起相关监管部门足够的重视。由于本文仅研究分析了上述3种重金属,这也使得RI相对较低,沉积物中重金属的污染状况还有待更进一步的调查研究。4. 结论
2010—2018年大亚湾表层沉积物中Cu、Pb和Zn的含量较稳定,空间分布上均呈现由近海岸向远海岸减少的趋势。Cu、Pb、Zn的含量呈显著正相关,表明3种重金属具有明显的同源性,沿岸工业企业排放以及澳头海域水产养殖等可能是近岸海域Cu、Pb和Zn的主要来源。
大亚湾表层沉积物中重金属污染程度为Zn>Cu>Pb。潜在生态风险评价表明,重金属的
$E_r^i $ 值呈现Cu>Pb>Zn,近十年来沉积物中Cu、Pb和Zn的污染状况并未随着经济的发展呈现出恶化的趋势,整体上3种重金属的潜在生态危险较轻微。 -
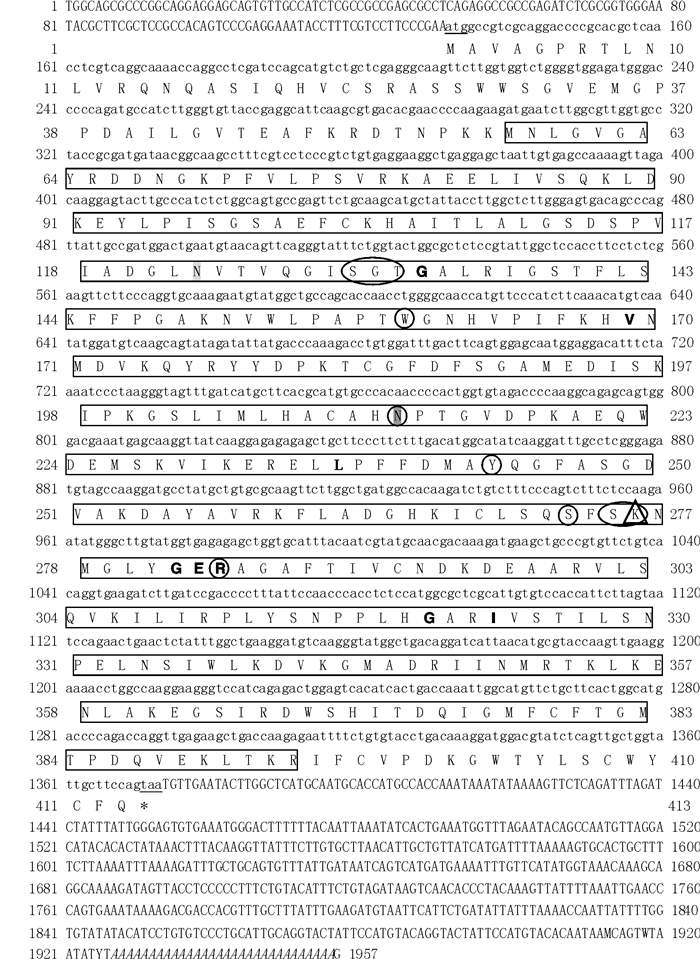
图 1 斑节对虾AST基因的核酸和氨基酸序列
两边每行标注的序号是指核苷酸和氨基酸的位置; 起始密码子(ATG)和终止密码子(TAA)用下划线标出; poly(A)尾巴用斜体字标出; 字体加粗标注为多肽结合位点; 灰色阴影标注为糖基化位点; 方框所示为谷AAT-like超家族结构域; 圆框中为磷酸吡哆醛结合位点; 三角框中为Lys催化残基。
Figure 1. Nucleotid and amino acid sequences of PmAST
The serial numbers on both sides of each row refer to the location of nucleotides and amino acids; start codon (ATG) and termination codon (TAA) are underlined; "*" indicates stop codon; the poly (a) signals are in italics; eight poly peptide sites are highlighted in bold; shadow area indicates two glycosylation sites; the completed AAT-I superfamily domains are shown in the box; ten PLP binding sites are represented by round frames; the catalytic residue is marked by a triangle.
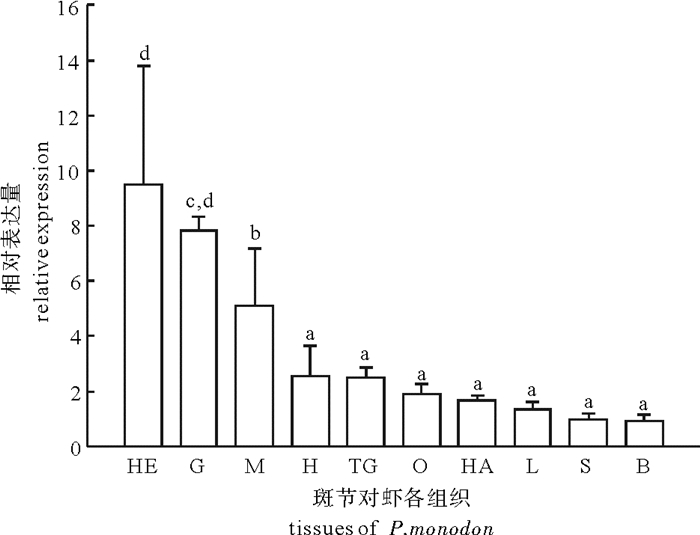
图 5 斑节对虾AST基因在各组织表达情况
HE.肝胰脏; G.鳃; M.肌肉; H.心脏; TG.胸神经; O:卵巢; HA.血淋巴L.淋巴; S.胃; B.脑; 图中数值为平均值±标准差(X ±SD), 小写字母不同表示差异性显著(P < 0.05)。
Figure 5. Expression of P.monodon AST in different tissues
HE.hepatopancreas; G.gill; M.muscle; H.heart; TG.thoracic ganglia; O.ovary; HA.haemolymph; L.lymph; S.stomach; B.brain; values (X ±SD) with different letters are significantly different from one another (P < 0.05).
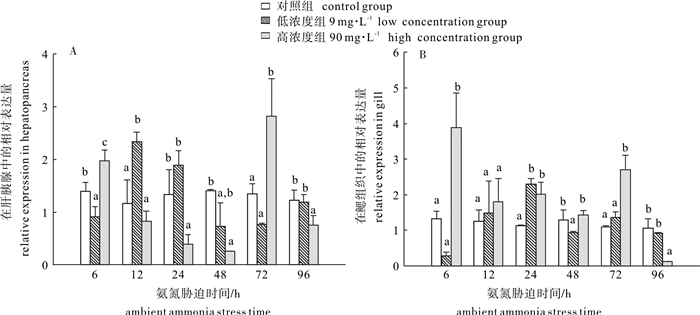
图 6 斑节对虾肝胰腺和腮中PmAST基因在氨氮胁迫过程中表达变化情况
图中数值为“平均值±标准差(X ±SD)”, 同一时间内小写字母不同表示实验组与对照组差异性显著(P < 0.05)。
Figure 6. Expression profiles of PmAST gene in P.monodon hepatopancreas and gill during ambient ammonia stress
Values (X ±SD) with different lowercase letters at the same time are significantly different with the control (P < 0.05).
表 1 斑节对虾AST基因克隆与表达分析所用引物序列
Table 1 Oligonucleotide primers used in the experiment
引物名称
primer引物序列(5′→3′)
primer sequence用途
functionTZ-F AATACGCTTCGCTCCGC 已知片段验证 TZ-R TTGCCTGCTTTGTTTACCAT TZS1 CCATCAGAGACTGGAGTCACATCA 3′RACE TZS2 TTCTGTGTACCTGACAAAGGATGG TZA1 CACCAACGCCAAGATTC 5′RACE TZA2 TGTCACGCTTGAATGCC β-actin-F AGTAGCCGCCCTGGTTGTAGA 内参基因 β-actin-R TTCTCCATGTCGTCCCAGT TZD-F AGCCCAGTTATTGCCGATG 荧光定量 PCR TZD-R GAAGGTGGAGCCAATACGGA -
[1] 李少飞, 何玉英, 李吉涛, 等.中国明对虾天门冬氨酸转氨酶基因的克隆及氨氮胁迫对其时空表达的影响[J].中国水产科学, 2014, 21(6):1125-1133. [2] 王镜岩, 朱胜庚, 徐长法.生物化学[M].3版.北京:高等教育出版社, 2002:306-308. [3] 潘劲劲. 天冬氨酸转氨酶与血小板比值在转氨酶两倍以下慢性乙型肝炎病毒感染患者中的临床应用评价[D]. 安徽: 安徽医科大学, 2014: 8-9. [4] 杜宗孝, 李富荣, 朴文花.血清天门冬氨酸氨基转移酶线粒体同工酶在酒精性肝病中的临床价值[J].检验医学, 2012, 27(9):732-735. http://med.wanfangdata.com.cn/Paper/Detail?id=PeriodicalPaper... [5] LIN L, ZENG X L, ZHANG J.Effect of profenofos poisoning on liver lipid peroxidation and liver function in rabbits[J].Chin J Clin Rehabilit, 2004, 8(21):4380-4381. https://www.researchgate.net/publication/286968630_Effect_of...
[6] 张彬彬.乙草胺对泥鳅肝脏谷丙转氨酶和谷草转氨酶的影响[J].江苏农业科学, 2009 (1):289-290. [7] 李波, 樊启学, 杨凯, 等.慢性氨氮胁迫对黄颡鱼摄食, 生长及血液指标的影响[J].应用与环境生物学报, 2011, 17(6):824-828. [8] 黄忠, 林黑着, 李卓佳, 等.复方中草药投喂策略对凡纳滨对虾生长, 消化及非特异性免疫功能的影响[J].南方水产科学, 2013, 9(5):37-43. http://www.wenkuxiazai.com/doc/4fb642ae7c1cfad6195fa7b2.html [9] MCKENNA M C, HOPKINS I B, LINDAUER S L, et al.Aspartate aminotransferase in synaptic and nonsynaptic mitochondria:differential effect of compounds that influence transient hetero-enzyme complex (metabolon) formation[J].Neurochem Int, 2006, 48(6):629-636. https://www.sciencedirect.com/science/article/pii/S0197018606000623
[10] MCCUTCHEON J P, von DOHLEN C D.An interdependent metabolic patchwork in the nested symbiosis of mealybugs[J].Curr Biol, 2011, 21(16):1366-1372. doi: 10.1016/j.cub.2011.06.051
[11] SCARAFFIA P Y, ISOE J, MURILLO A, et al.Ammonia metabolism in Aedes aegypti[J].Insect Biochem Molec, 2005, 35(5):491-503. doi: 10.1016/j.ibmb.2005.01.012
[12] CHEN J, ZHOU F, HUANG J, et al.Ammonia and salinity tolerance of Penaeus monodon across eight breeding families[J].SpringerPlus, 2016, 5(1):1. doi: 10.1186/s40064-015-1659-2
[13] 韩春艳, 郑清梅, 陈桂丹, 等.氨氮胁迫对奥尼罗非鱼非特异性免疫的影响[J].南方水产科学, 2014, 10(3):47-52. [14] 李少飞. 中国对虾氨氮代谢酶基因的cDNA克隆及其在氨氮解毒代谢过程中的作用[D]. 大连: 大连海洋大学, 2014: 28-30. [15] 刘胜男, 潘鲁青, 刘茂琪.氨氮胁迫对三疣梭子蟹解毒代谢关键基因表达的影响[J].海洋湖沼通报, 2015 (2):97-104. http://www.cqvip.com/QK/95761X/201502/666585651.html [16] 岳峰. 三疣梭子蟹在氨氮胁迫下免疫应答与解毒代谢机制的研究[D]. 青岛: 中国海洋大学, 2010: 11-12. [17] 胡志国, 刘建勇, 袁瑞鹏, 等.凡纳滨对虾高氨氮和低溶氧抗逆性状的杂交配合力分析[J].南方水产科学, 2016, 12(1):43-49. http://www.cqvip.com/QK/60642B/201601 [18] 肖炜, 李大宇, 徐杨, 等.慢性氨氮胁迫对吉富罗非鱼幼鱼生长, 免疫及代谢的影响[J].南方水产科学, 2015, 11(4):81-87. http://www.cqvip.com/QK/60642B/201504/665703414.html [19] 黄建华, 李永, 杨其彬, 等.斑节对虾家系氨氮耐受性的比较[J].南方水产科学, 2012, 8(6):37-43. http://www.oalib.com/paper/4365344 [20] 郑微云, 翁思琪.环境毒理学概论[M].厦门:厦门大学出版社, 1993:57-60. [21] ZANG W L, XU X C, DAI X L, et al.Toxic effects of Zn2+, Cu2+, Cd2+ and NH3 on Chinese prawn[J].Chin J Oceanol Limnol, 1993, 3:254-259.
[22] 荆晓丽. 青岛文昌鱼谷丙转氨酶基因克隆, 表达和功能研究[D]. 青岛: 中国海洋大学, 2010: 56-61. [23] MEHTA P K, HALE T I, CHRISTEN P.Aminotransferases:demonstration of homology and division into evolutionary subgroups[J].Eur J Biochem, 1993, 214(2):549-561. doi: 10.1111/ejb.1993.214.issue-2
[24] 陈玉春, 顾雪飞, 刘敏.5种中草药对鲤血清谷丙转氨酶, 谷草转氨酶及红细胞过氧化氢酶活性的影响[J].淡水渔业, 2007, 37(5):11-13. http://www.wenkuxiazai.com/doc/cd1e180f866fb84ae45c8d81.html [25] 赵杰, 谷子林, 崔青曼, 等."克白克"对中国对虾体内琥珀酸脱氢酶, 磷酸甘油三酯和谷草转氨酶活性的影响[J].中国饲料, 2002(3):14. http://industry.wanfangdata.com.cn/dl/Magazine?magazineId=zgsl&... [26] REGNAULT M.Nitrogen excretion in marine and fresh-water crustacea[J].Biol Rev, 1987, 62(1):1-24. https://www.researchgate.net/publication/229992875_Nitrogen...
[27] CHEN J C, LIN C Y.Responses of oxygen consumption, ammonia-N excretion and urea-N excretion of Penaeus chinensis exposed to ambient ammonia at different salinity and pH levels[J].Aquaculture, 1995, 136(3):243-255. https://es.scribd.com/document/153402238/2009-Rational-pdf
[28] CHEN J M, CHEN J C.Study on the free amino acid levels in the hemolymph, gill, hepatopancreas and muscle of Penaeus monodon exposed to elevated ambient ammonia[J].Aquat Toxicol, 2000, 50(1):27-37.
[29] WALTON D J.Stereochemistry of reduction of D-glyceraldehyde catalyzed by a nicotinamide adenine dinucleotide phosphate dependent dehydrogenase from skeletal muscle[J].Biochemistry, 1973, 12(18):3472-3478. doi: 10.1021/bi00742a018
[30] 李永. 斑节对虾人工选育及氨氮对其免疫, 解毒代谢影响[D]. 上海: 上海海洋大学, 2012: 9. [31] 曾媛媛, 陈曦飞, 艾春香, 等. 氨氮胁迫对拟穴青蟹血淋巴生理生化指标及鳃和肝胰腺显微结构的影响[C]//中国甲壳动物学会第十一届年会暨学术研讨会论文摘要集. 厦门: 中国海洋湖沼学会, 2011: 107. -
期刊类型引用(4)
1. 刘倩,刘永,张林宝,陈海刚,张喆,田斐,王学锋. 基于综合生物标志物响应法的渔港重金属污染风险评价. 渔业科学进展. 2024(02): 28-38 .  百度学术
百度学术
2. 王立明,孙珊,李志林,金晓杰,赵玉庭,于潇潇,刘继晨. 光催化处理海洋重金属污染的分析及应用. 工业催化. 2024(03): 20-28 .  百度学术
百度学术
3. 冯雪,佟飞,袁华荣,赵学乾,陈丕茂. 外伶仃海洋牧场附近海域沉积物重金属分布特征及生态风险评价. 南方水产科学. 2024(05): 91-102 .  本站查看
本站查看
4. 孙业皎,黄翠玲,隋琪,娄安刚,夏斌,张旭志,赵信国,陈碧鹃,曲克明. 桑沟湾表层海水中重金属含量季节变化及污染分析. 海洋环境科学. 2021(05): 752-759 .  百度学术
百度学术
其他类型引用(1)




 下载:
下载:











 粤公网安备 44010502001741号
粤公网安备 44010502001741号
