Advances in vitellogenin research of aquatic animals
-
摘要:
卵黄蛋白原(vitellogenin,VTG)是卵生动物卵黄中主要成分的前体。文章简述了水产动物如鱼类、两栖类及甲壳类动物中卵黄蛋白原基因的鉴定及其基因家族的分类及进化研究,介绍了VTG分子的生物学新功能,以及VTG作为标记分子在环境雌激素监测、反映雌性动物性腺成熟度、区分雌雄性别等方面的应用,以期为VTG在水产动物中的研究以及繁殖中的应用提供参考。
Abstract:Vitellogenins (VTG) are precursors of major components of female yolk proteins in oviparous animals. The paper briefly reviews the VTG gene characterization in aquatic animals such as fish, amphibian and crustaceans as well as the classification and evolution of their gene families, introduces the new physiological functions of VTG, and summarizes their usages as biomarker in monitoring environmental endocrine disruption, predictive factor of gonad maturity and effective marker in gender discrimination. Thus, the review provides references for researches on VTG in aquatic animals and breeding of aquatic animals.
-
Keywords:
- vitellogenin /
- new physiological function /
- biological marker /
- evolution
-
近十几年来,我国对虾养殖业发展迅速,取得了较大的经济效益和社会效益,但同时也由于从业者的养殖理念及管理技术水平还存在一定的局限,从而造成了养殖水体富营养化、养殖生态环境失衡、对虾病害频发和产品质量下降等一系列制约对虾养殖业和谐发展的瓶颈性问题。如何更新理念,有效利用生物、生态学技术提高对虾养殖管理水平,促进对虾养殖的可持续发展,这已日渐成为许多科研工作者的研究重点。微藻技术是一项有机结合藻类生物学及藻类环境生态学的新兴生物技术,它以水体中的浮游微藻为对象,以运用微藻生理、生态特点调控、优化养殖生态环境为目的。相关研究表明,浮游微藻作为对虾养殖池塘生态系统中的重要组成部分之一,与养殖池塘的水体质量、养殖对虾的健康水平以及池塘生态系统的平衡与否具有重要的相关性[1-5]。本文就当前在水产养殖领域中的微藻生物技术的相关研究进行了系统的综述,以期为进一步探讨构建以微藻生物技术为核心的池塘藻相调控技术,并以此维持对虾养殖池塘生态系统的动态平衡,降低对虾养殖对周边水域环境的负面影响提供参考。
1. 对虾养殖池中浮游微藻的生态功能
浮游微藻对对虾养殖池的物质循环和能量流动具有举足轻重的作用,它对于维持虾池生态系统的正常功能,稳定池塘环境是不可或缺的。有研究表明,对虾疾病的爆发与水体中浮游微藻群落结构的变化具有直接或间接的关系,虾池中浮游微藻的种类和数量尤其是赤潮生物的类群和数量与对虾发病程度有正相关性;而其多样性指数则与对虾病害的发生呈负相关[1, 6-7]。其次,浮游微藻的种群组成、种群密度与水体理化因子密切相关,虾池中浮游微藻的种类、数量可直接影响水体理化因子的变化[8-11]。优良的浮游微藻藻相在种群稳定、生物量持续增长的过程中,可促进水体中营养盐的分解与转化,减少并消除氨氮、亚硝酸氮、有机污染物等多种有毒物质;而且还可通过光合作用产氧而增加养殖水体的溶解氧,促进水体中富含的耗氧性有机质的氧化分解。一旦水体中优良微藻藻相生态平衡被打破,有害微藻过度繁殖或环境中的微藻种类过于单一,则既不利于促进养殖生态系统的良性循环,保持养殖环境的相对稳定,也不利于维持良好的水质环境,进而严重影响养殖对虾的健康水平,可见,从某种意义上来说,养殖环境的优良与否是引发对虾产生应激反应和抗病力下降的主要诱因[1, 6-7]。
因此,笔者认为有必要从微藻种类、数量、物种多样性指数等因子的变化,以及它对虾池水质环境,乃至整个虾池生态系统的影响进行系统的研究与分析,从而筛选出生产性能良好、环境兼容性强的优良藻株,用以构建优良藻相,优化虾池中浮游微藻的藻相结构,进而建立以微藻生物技术为核心的池塘藻相调控技术。这对维持对虾养殖池塘生态系统的动态平衡,促进对虾的健康生长具有重要的现实意义。
2. 虾池中浮游微藻群落结构的特点
2.1 虾池中浮游微藻群落结构的特点
根据国内外学者以往对虾池中浮游微藻群落结构的研究结果[7, 12-15],其主要特征有如下几点:(1)群落组成包括浮游性种类和底栖性种类,但总体而言其种类数目要少于外海水域;(2)群落中优势种单一,优势度高,耐污性种类较多,有些为赤潮或有毒、有害赤潮生物;(3)虾池及所纳入的养殖水体中藻类的生物量远高于沿岸水域,但种类要少于沿岸水域;(4)虾池中藻类细胞数量远高于所引入水源中的细胞数量;(5)养殖后期,虾池中耐污种类和赤潮种类增多,藻类生物的群落演替具有突发性,时间短,速度快,群落结构极不稳定的特点;(6)虾池中藻类的多样性指数基本可代表其水质情况即水体富营养化程度。
对于不同的虾池在不同的养殖时间,其浮游微藻群落的多样性也有所不同。在凡纳滨对虾淡化养殖池中,一般有2~3个优势种,环境中的优势种越少其优势度就越高[8, 13]。就生产性能而言,对虾养殖生产中通常以绿藻、硅藻为优势种的池塘为好,其水质稳定,病害少,对虾生长亦较好;以蓝藻为优势的水体中,对虾生长缓慢而且容易引发病害[1]。因此,在养殖过程中以培养绿藻为主的绿色水系较好,究其原因可能是在绿藻为主的绿色水系中藻类种群较多,水体生态系相对稳定,容易保持池水的“活、爽”,而以硅藻为主的褐色水系藻类单一,不及绿色水系稳定,容易因气候变化而变动,从而引起对虾产生应激发应[1, 14]。
但也有学者提出不同的看法,有的学者认为在虾池生态环境中浮游藻类的生物量优势种的生态功能较数量优势种的更为重要。有些种类虽然个体数量的优势度突出,但其个体小,生物量少,对浮游微藻群落和养殖环境稳定性的维持作用并不大,而有些种类的数量并不占优势,但其个体大,生物量高,光合作用强,对维持微藻群落及养殖环境稳定性具有重要的作用。并且,有些优势种藻类可同时出现,有些则表现为相互抑制。例如在以蓝藻为优势种的虾池中,硅藻、隐藻和绿藻不易形成优势种,可能是蓝藻在富营养化水体环境中更具有竞争优势,对其它藻类产生抑制[13]。估计这主要可能有营养、空间等方面的竞争,以及其所分泌的胞外物质对其它藻类有抑制性作用。因此,在构建优良微藻藻相时要充分考虑各藻种间的相互关系,从而进行科学合理地筛选、组合和优化。
2.2 养殖水体富营养化与藻类多样性指数的相关性
有学者研究认为虾池水体富营养化的主要特征是过量的营养盐和有机物引起浮游藻类的群落结构发生变化,多样性指数降低。当指数低于一定的域值时,水质会出现不同程度的变色、发黑等恶化的表征[5, 16]。有研究结果表明,养殖初期一般以绿藻、硅藻为主,随着养殖时间增加养殖水体开始出现富营养化,浮游植物的种类和生物量也随之增多,到养殖后期水体富营养化严重,优势种基本为裸藻、蓝藻等耐污性强的藻类[14]。还有学者提出若从对虾生长情况来看,过低的多样性指数容易导致对虾不同程度地出现病症和死亡[7]。可见,其多样性指数不但能从本质上反映出养殖水质的优劣程度,还与对虾发病程度呈负相关。所以在对虾养殖过程中,应该尽量保持环境的相对稳定,避免采用那些容易引起环境突发性变化的调控手段,而应建立、维持优良的微藻藻相,以稳定养殖环境的生态平衡。
3. 浮游藻类利用虾池水体中营养盐的研究
有研究表明,养殖水体中各水化因子对浮游藻类生长影响的次序依次为磷酸盐,氨氮,铁离子,亚硝氮,酸碱度,硝酸氮,温度,锰离子,溶解氧[1]。可见,氮、磷营养盐对藻类的生长具有极其重要的影响。但不同种类的微藻对不同的营养盐浓度和氮、磷配比有不同的要求。有数据显示,在平均盐度为20.0的精养虾池中,其平均总氮、磷和铁的浓度分别为0.97、0.05和0.15 mg·L-1[8],而海洋浮游藻类对无机氮和无机磷的最适浓度下限分别为79.9和18.0 μg·L-1[14, 17]。因此,笔者认为有必要根据精养虾池的水质情况和浮游微藻对营养盐的需求进行系统的调查与分析,为合理构建、调控、优化养殖水体的藻相结构提供适宜的营养参数和生态参数。
还有学者提出就同一水体而言,有时其营养盐的峰值与浮游藻类的繁殖峰期会存在一个时间差,即两者浓度和含量的变化是动态的,且具有一定的变化规律。虾池是一个封闭半封闭的人工生态系统,其营养盐浓度和藻类生物量都可能因为受到人为的以及生物或非生物的各种环境因素的影响[18],而在短时间内发生较大的波动,因而对两者之间的定量关系进行研究具有一定的难度。目前大部分研究浮游藻类的营养盐需求时,通常采用以下2种方法:(1)研究藻类对营养盐的吸收速率和营养盐限制藻类的增长速率;(2)利用化学计量限制法把培养环境中的营养盐之和、各营养盐的配比,以及藻类生物量的结构进行系统的相关性研究,通过对营养盐的吸收比值进行比较,从而确定何种营养盐首先被消耗利用[19-20]。
在对对虾养殖池塘生态环境进行综合调查与分析的基础上,笔者认为目前迫切需要对养殖环境中的微藻种类进行研究,以确定哪些种类对稳定环境更为有利,进而筛选出生产性能良好、环境兼容性强的优良藻种,进行科学搭配、组合和优化,构建出优良的藻相,但目前国内外学者对构建优良藻相,调控精养虾池养殖环境的相关研究还鲜见报道。
4. 微藻技术在对虾养殖中的应用与展望
对虾养殖业是我国渔业的重要组成部分,近十几年来发展极为迅速,目前,我国的对虾生产以集约化、半集约化的池塘养殖为主体,由于养殖密度的提高,加之养殖过程中对水质调控技术的不合理,以致造成水体富营养化、养殖生态环境失衡、对虾病害频发和产品质量下降等一系列严重问题。显然,寻求安全、环保、有效的措施,维持虾池生态系平衡,营造良好的养殖生态环境,促进对虾的健康生产,同时实施封闭与半封闭的科学管理,是生产质量安全的养殖对虾产品,维系我国对虾养殖业可持续发展的关键。笔者认为可应用微藻生物技术,在对虾集约化养殖池、半集约化养殖土池中筛选、培育优良微藻,构建藻相,用以吸收水体中富含的氮、磷,并通过光合作用产氧而增加水体中的溶解氧,氧化分解水中的耗氧性有机质,从而达到促进池塘环境的物质循环,优化养殖水体环境,降低集约化养殖的自源性污染,进而减轻其对周边水源环境的负面影响[2, 21]。
为系统建立高效的微藻藻相技术,还需从以下几个方面进行探索与研究。(1)应在不同养殖模式、不同养殖阶段、不同季节下,对对虾养殖池塘中的浮游微藻的种群结构进行系统的调查,摸清池塘中浮游藻类种群组成;(2)根据国内外学者对环境中浮游藻类群落结构的特点,自然水体中藻类的生物学特点,及温度、盐度、pH、营养盐等生态因子对其生长繁殖的影响等各方面的研究结果,并充分考虑对虾养殖生产中的实际情况,进而筛选出多株生产性能良好、环境兼容性强的优良藻种,构建优良藻相;再者,还需在摸清各种微藻个体培养的营养参数和生态参数的基础上,进一步优化藻相结构,并就构建藻相群体培养的营养需求水平和生态条件进行有效的摸索,从而为将优良微藻藻相调控系统技术有效应用于养殖生产实践中提供有力的技术支撑。
-
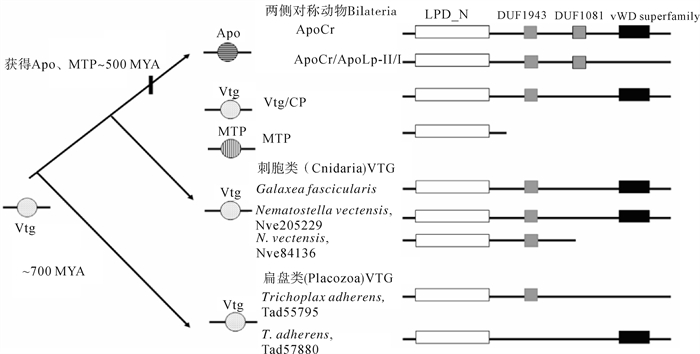
图 1 LLTP超家族的进化及各家族成员的功能结构域组成示意图
白框(LPD_N). 脂蛋白氨基端;灰框(DUF1943和DUF1081). DUF1943和DUF1081功能结构域;黑框(vWD superfamily). vonWillebrand factor type D结构域(修自[17])
Figure 1. Schematic figure of evolutionary path of LLTP superfamily and structure of VTG, Apo and MTP families
white boxes (LPD_ N). domains of lipoprotein amino terminal regions; grey boxes (DUF1943 and DUF1081). DUF1943 and DUF1081 domains; black boxes (vWD superfamily). vonWillebrand factor type D domains (revised from Reference[17])
-
[1] PROWSE T A, BYRNE M. Evolution of yolk protein genes in the Echinodermata[J]. Evol Dev, 2012, 14(2): 139-151. doi: 10.1111/j.1525-142X.2012.00531.x
[2] HAYAKAWA H, ANDOH T, WATANABE T. Precursor structure of egg proteins in the coral Galaxea fascicularis[J]. Biochem Biophys Res Commun, 2006, 344(1): 173-180. doi: 10.1016/j.bbrc.2006.03.116
[3] BABIN P J. Conservation of a vitellogenin gene cluster in oviparous vertebrates and identification of its traces in the platypus genome[J]. Gene, 2008, 413(1/2): 76-82. doi: 10.1016/j.gene.2008.02.001
[4] 马杰, 张士璀. 卵黄蛋白的结构和功能[J]. 鲁东大学学报: 自然科学版, 2012, 28(3): 252-260. doi: 10.3969/j.issn.1673-8020.2012.03.014 [5] TINGAUD-SEQUEIRA A, KNOLL-GELLIDA A, ANDRE M, et al. Vitellogenin expression in white adipose tissue in female teleost fish[J]. Biol Reprod, 2012, 86(2): 38. doi: 10.1095/biolreprod.111.093757
[6] LIU K C, WU R S S, GE W. Luteinizing hormone receptor (lhcgr) as a marker gene for characterizing estrogenic endocrine-disrupting chemicals in zebrafish ovarian follicle cells[J]. Gen Comp Endocrinol, 2013, 192: 89-94. doi: 10.1016/j.ygcen.2013.06.023
[7] JIA X, CHEN Y, ZOU Z, et al. Characterization and expression profile of vitellogenin gene from Scylla paramamosain[J]. Gene, 2013, 520(2): 119-130. doi: 10.1016/j.gene.2013.02.035
[8] AGNESE M, VERDERAME M, De MEO E, et al. A network system for vitellogenin synthesis in the mussel Mytilus galloprovincialis (L. )[J]. J Cell Physiol, 2013, 228(3): 547-555. doi: 10.1002/jcp.24161
[9] READING B J, HIRAMATSU N, SULLIVAN C V. Disparate binding of three types of vitellogenin to multiple forms of vitellogenin receptor in white perch[J]. Biol Reprod, 2011, 84(2): 392-399. doi: 10.1095/biolreprod.110.087981
[10] TSANG W S, QUACKENBUSH L S, CHOW B K, et al. Organization of the shrimp vitellogenin gene: evidence of multiple genes and tissue specific expression by the ovary and hepatopancreas[J]. Gene, 2003, 303: 99-109. doi: 10.1016/S0378-1119(02)01139-3
[11] 刘春, 李凯彬, 耿冬雨, 等. 剑尾鱼2种卵黄蛋白原全长cDNA的克隆及序列分析[J]. 中国水产科学, 2010, 17(1): 31-43. https://d.wanfangdata.com.cn/periodical/zgsckx201001004 [12] FINN R N, KRISTOFFERSEN B A. Vertebrate vitellogenin gene duplication in relation to the "3R hypothesis": correlation to the pelagic egg and the oceanic radiation of teleosts[J]. PLoS ONE, 2007, 2(1): e169. doi: 10.1371/journal.pone.0000169
[13] WU L T, HUI J H, CHU K H. Origin and evolution of yolk proteins: expansion and functional diversification of large lipid transfer protein superfamily[J]. Biol Reprod, 2013, 88(4): 102. https://xueshu.baidu.com/usercenter/paper/show?paperid=e6e67e61b71824d68e8f3002c3baccef&site=xueshu_se
[14] 李昀. 栉孔扇贝(Chlamys farreri)两个卵黄蛋白原转录本的发育表达图式[D]. 青岛: 中国海洋大学, 2011. 10.7666/d.y1926674 [15] LAFLEUR G J, Jr., RALDUA D, FABRA M, et al. Derivation of major yolk proteins from parental vitellogenins and alternative processing during oocyte maturation in Fundulus heteroclitus[J]. Biol Reprod, 2005, 73(4): 815-824. doi: 10.1095/biolreprod.105.041335
[16] AVARRE J C, LUBZENS E, BABIN P J. Apolipocrustacein, formerly vitellogenin, is the major egg yolk precursor protein in decapod crustaceans and is homologous to insect apolipophorin Ⅱ/Ⅰ and vertebrate apolipoprotein B[J]. BMC Evol Biol, 2007, 7: 3. doi: 10.1186/1471-2148-7-3
[17] HAYWARD A, TAKAHASHI T, BENDENA W G, et al. Comparative genomic and phylogenetic analysis of vitellogenin and other large lipid transfer proteins in metazoans[J]. FEBS Lett, 2010, 584(6): 1273-1278. doi: 10.1016/j.febslet.2010.02.056
[18] BROOKS J M, WESSEL G M. The major yolk protein in sea urchins is a transferrin-like, iron binding protein[J]. Dev Biol, 2002, 245(1): 1-12. doi: 10.1006/dbio.2002.0611
[19] SAPPINGTON T W. The major yolk proteins of higher diptera are homologs of a class of minor yolk proteins in lepidoptera[J]. J Mol Evol, 2002, 55(4): 470-475. doi: 10.1007/s00239-002-2342-0
[20] WILLIAMS V N, READING B J, HIRAMATSU N, et al. Multiple vitellogenins and product yolk proteins in striped bass, Morone saxatilis: molecular characterization and processing during oocyte growth and maturation[J]. Fish Physiol Biochem, 2014, 40(2): 395-415. doi: 10.1007/s10695-013-9852-0
[21] ZHANG S, SUN Y, PANG Q, et al. Hemagglutinating and antibacterial activities of vitellogenin[J]. Fish Shellfish Immunol, 2005, 19(1): 93-95. doi: 10.1016/j.fsi.2004.10.008
[22] LIU Q H, ZHANG S C, LI Z J, et al. Characterization of a pattern recognition molecule vitellogenin from carp (Cyprinus carpio)[J]. Immunobiology, 2009, 214(4): 257-267. doi: 10.1016/j.imbio.2008.10.003
[23] LI Z, ZHANG S, LIU Q. Vitellogenin functions as a multivalent pattern recognition receptor with an opsonic activity[J]. PLoS ONE, 2008, 3(4): e1940. doi: 10.1371/journal.pone.0001940
[24] LI Z, ZHANG S, ZHANG J, et al. Vitellogenin is a cidal factor capable of killing bacteria via interaction with lipopolysaccharide and lipoteichoic acid[J]. Mol Immunol, 2009, 46(16): 3232-3239. doi: 10.1016/j.molimm.2009.08.006
[25] WANG S, WANG Y, MA J, et al. Phosvitin plays a critical role in the immunity of zebrafish embryos via acting as a pattern recognition receptor and an antimicrobial effector[J]. J Biol Chem, 2011, 286(25): 22653-22664. doi: 10.1074/jbc.M111.247635
[26] TONG Z, LI L, PAWAR R, et al. Vitellogenin is an acute phase protein with bacterial-binding and inhibiting activities[J]. Immunobiology, 2010, 215(11): 898-902. doi: 10.1016/j.imbio.2009.10.001
[27] GARCIA J, MUNRO E S, MONTE M M, et al. Atlantic salmon (Salmo salar L. ) serum vitellogenin neutralises infectivity of infectious pancreatic necrosis virus (IPNV)[J]. Fish Shellfish Immunol, 2010, 29(2): 293-297. doi: 10.1016/j.fsi.2010.04.010
[28] SUN C, HU L, LIU S, et al. Antiviral activity of phosvitin from zebrafish Danio rerio[J]. Dev Comp Immunol, 2013, 40(1): 28-34. doi: 10.1016/j.dci.2012.12.009
[29] AKASAKA M, HARADA Y, SAWADA H. Vitellogenin C-terminal fragments participate in fertilization as egg-coat binding partners of sperm trypsin-like proteases in the ascidian Halocynthia roretzi[J]. Biochem Biophys Res Commun, 2010, 392(4): 479-484. doi: 10.1016/j.bbrc.2010.01.006
[30] AKASAKA M, KATO K H, KITAJIMA K, et al. Identification of novel isoforms of vitellogenin expressed in ascidian eggs[J]. J Exp Zool B Mol Dev Evol, 2013, 320(2): 118-128. doi: 10.1002/jez.b.22488
[31] WANG Y, BRENT C S, FENNERN E, et al. Gustatory perception and fat body energy metabolism are jointly affected by vitellogenin and juvenile hormone in honey bees[J]. PLoS Genet, 2012, 8(6): e1002779. doi: 10.1371/journal.pgen.1002779
[32] SEEHUUS S C, NORBERG K, GIMSA U, et al. Reproductive protein protects functionally sterile honey bee workers from oxidative stress[J]. Proc Natl Acad Sci USA, 2006, 103(4): 962-967. doi: 10.1073/pnas.0502681103
[33] CURRYLOW A F, TIFT M S, MEYER J L, et al. Seasonal variations in plasma vitellogenin and sex steroids in male and female eastern box turtles, Terrapene carolina carolina[J]. Gen Comp Endocrinol, 2013, 180: 48-55. doi: 10.1016/j.ygcen.2012.11.005
[34] 王凤, 张颖, 佟广香, 等. 哲罗鲑生殖周期中血清卵黄蛋白原浓度的ELISA检测[J]. 上海海洋大学学报, 2009, 18(6): 673-679. https://d.wanfangdata.com.cn/periodical/ChpNaW5lclBlcmlvZGljYWxDSEkyMDIyMDkwORIRc2hzY2R4eGIyMDA5MDYwMDYaCDZzY2J5MTlk [35] FERRE L E, MEDESANI D A, GARCIA C F, et al. Vitellogenin levels in hemolymph, ovary and hepatopancreas of the freshwater crayfish Cherax quadricarinatus (Decapoda: Parastacidae) during the reproductive cycle[J]. Rev Biol Trop, 2012, 60(1): 253-261. https://pubmed.ncbi.nlm.nih.gov/22458222/
[36] BAUMANN L, HOLBECH H, KEITER S, et al. The maturity index as a tool to facilitate the interpretation of changes in vitellogenin production and sex ratio in the fish sexual development test[J]. Aquat Toxicol, 2013, 128/129: 34-42. doi: 10.1016/j.aquatox.2012.11.016
[37] CHATAKONDI N G, KELLY A M. Oocyte diameter and plasma vitellogenin as predictive factors to identify potential channel catfish, Ictalurus punctatus, suitable for induced spawning[J]. J World Aquac Soc, 2013, 44(1): 115-123. doi: 10.1111/jwas.12001
[38] CHU-KOO F, DUGUÉ R, AGUILAR M A, et al. Gender determination in the Paiche or Pirarucu (Arapaima gigas) using plasma vitellogenin, 17β-estradiol, and 11-ketotestosterone levels[J]. Fish Physiol Biochem, 2009, 35(1): 125-136. doi: 10.1007/s10695-008-9211-8
[39] 齐茜, 曲秋芝, 张颖, 等. 小体鲟血清卵黄蛋白原和Ca2+浓度与卵巢发育的关系[J]. 中国水产科学, 2009, 16(6): 967-974. doi: 10.3321/j.issn:1005-8737.2009.06.018 [40] BURGMEIER N G, UNGER S D, MEYER J L, et al. Health and habitat quality assessment for the eastern hellbender (Cryptobranchus alleganiensis alleganiensis) in Indiana, USA[J]. J Wildl Dis, 2011, 47(4): 836-848. doi: 10.7589/0090-3558-47.4.836
[41] KOHN Y Y, LOKMAN P M, KILIMNIK A, et al. Sex identification in captive hapuku (Polyprion oxygeneios) using ultrasound imagery and plasma levels of vitellogenin and sex steroids[J]. Aquaculture, 2013, 384/385/386/387: 87-93. https://www.sciencedirect.com/science/article/pii/S0044848613000021
[42] 杨丽丽, 张晶, 方展强, 等. 雌二醇、壬基酚、多氯联苯、镉和锌及其混合物对唐鱼的雌激素效应比较[J]. 水产学报, 2011, 35(6): 838-845. doi: 10.3724/SP.J.1231.2011.17247 [43] 王宏元, 巨荣菊, 白瑶, 等. 壬基酚对雄性中国林蛙肝细胞中雌激素受体和卵黄蛋白原的影响[J]. 陕西师范大学学报: 自然科学版, 2011, 39(6): 54-59. https://d.wanfangdata.com.cn/periodical/sxsfdxxb201106015 [44] 谢勇平, 方展强. 利用食蚊鱼目标基因转录水平评价东莞寒溪河雌/雄激素物质污染现状[J]. 水生生物学报, 2013, 37(4): 691-697. doi: 10.7541/2013.81 [45] 顾海龙, 陈彩芳, 林志华等. 水生生物卵黄蛋白原在内分泌干扰物检测中的应用[J]. 宁波大学学报: 理工版, 2013(2): 12-16. https://d.wanfangdata.com.cn/periodical/nbdxxb-lg201302005 [46] 宋双双, 安立会, 郑丙辉, 等. 浑河流域野生鲫鱼卵黄蛋白原基因表达[J]. 生态毒理学报, 2013, 8(1): 121-129. doi: 10.7524/AJE.1673-5897.20121011002 [47] 张艳珍, 陈细华, 危起伟, 等. 中华鲟血清卵黄蛋白原水平的初步观察[J]. 淡水渔业, 2008, 38(5): 10-14. doi: 10.3969/j.issn.1000-6907.2008.05.003 [48] 李育培, 刁晓明, 盛晓洒, 等. 瓦氏黄颡鱼(Pelteobagrus vachelli)卵黄蛋白原的纯化、性质鉴定及ELISA检测方法的建立[J]. 海洋与湖沼, 2010, 41(1): 91-98. doi: 10.11693/hyhz201001013013 [49] PECK K A, LOMAX D P, OLSON O P, et al. Development of an enzyme-linked immunosorbent assay for quantifying vitellogenin in Pacific salmon and assessment of field exposure to environmental estrogens[J]. Environ Toxicol Chem, 2011, 30(2): 477-486. https://pubmed.ncbi.nlm.nih.gov/21038437/
[50] WATTS M, PANKHURST N W, PRYCE A, et al. Vitellogenin isolation, purification and antigenic cross-reactivity in three teleost species [J]. Comp Biochem Physiol B, 2003, 134(3): 467-476. doi: 10.1016/S1096-4959(02)00288-9
[51] 程翔, 蔡生力, 刘红, 等. 中国明对虾卵黄蛋白原mRNA在卵巢和肝胰腺中表达量的实时荧光定量PCR检测[J]. 上海海洋大学学报, 2012, 21(1): 1-6. https://www.cqvip.com/qk/90212a/2012001/40504437.html [52] 陈浩, 杨健, 王跃祥, 等. 卵黄蛋白原1(vtg1)启动子调控绿色荧光蛋白表达的转基因斑马鱼的构建[J]. 生物化学与生物物理进展, 2006, 33(10): 965-970. doi: 10.3321/j.issn:1000-3282.2006.10.009 [53] 武佳. 利用唐鱼卵黄蛋白原启动子检测环境雌激素的研究[D]. 广州: 华南师范大学, 2011. https://d.wanfangdata.com.cn/thesis/Y1984529 [54] OM A D, JASMANI S, ISMAIL N, et al. Application MALDI TOF on protein identification of vitellogenin in giant grouper (Epinephelus lanceolatus)[J]. Fish Physiol Biochem, 2013, 39(5): 1277-1286. doi: 10.1007/s10695-013-9782-x
[55] RIDEOUT R M, MORGAN M J, LAMBERT Y, et al. Oocyte development and vitellogenin production in Northwest Atlantic Greenland halibut Reinhardtius hippoglossoides[J]. J Northw Atl Fish Sci, 2012, 44: 15-30. doi: 10.2960/J.v44.ms679
[56] HAVUKAINEN H, MVNCH, D, BAUMANN A, et al. Vitellogenin recognizes cell damage through membrane binding and shields living cells from reactive oxygen species[J]. J Biol Chem, 2013, 288(39): 28369-28381. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3784755/
[57] ZHANG S, WANG S, LI H, et al. Vitellogenin, a multivalent sensor and an antimicrobial effector[J]. Int J Biochem Cell Biol, 2011, 43(3): 303-305. https://www.sciencedirect.com/science/article/pii/S135727251000381X



 下载:
下载:
 粤公网安备 44010502001741号
粤公网安备 44010502001741号
