Preparation of PLGA microparticles containing GBS and their release characteristics in vitro
-
摘要:
以乳酸-羟基乙酸共聚物(PLGA)为材料,采用复乳溶剂挥发法制备无乳链球菌(Streptococcus agalactiae)全菌及超声破碎后的上清微球疫苗。显微镜观察显示随着PLGA质量浓度、PVA(聚乙烯醇)质量浓度和外水相体积的增加,上清微球平均粒径均随之增大。最终确定上清微球制备条件为PLGA质量浓度25 mg · mL-1、PVA质量浓度1 mg · mL-1、外水相体积20 mL。全菌微球制备条件与上述的相比,仅PVA质量浓度调整为2 mg · mL-1。扫描电镜观察显示全菌和上清微球平均粒径分别为9.4 μm和3.9 μm,微球均呈球形。BCA(二喹啉甲酸)法分析显示包封率分别为68.07%和63.49%;载药量分别为5.49×108个· mg-1和3.58%;28 d体外累积释放量分别为64.2%和82.8%。
Abstract:We prepared polylactic-co-glyconlicacid (PLGA) microparticles containing Streptococcus agalactiae (Group B streptococcus, GBS) whole cell and supernatant after ultrasonication by double emulsion-solvent evaporation method. With increasing PLGA concentration, emulsifier concentration and volume of outer water phase, the average diameter of microparticles containing supernatant increased. We used 25 mg · mL-1 of PLGA, 1 mg · mL-1 of emulsifier concentration and 20 mL of outer water phase for preparation of microparticles containing supernatant. The preparation parameters were similar for GBS whole cell microparticles except that the emulsifier concentration was 2 mg · mL-1. The result by scanning electron microscope (SEM) shows that the average diameters of GBS whole cell and supernatant microparticles were 9.4 μm and 3.9 μm, respectively, and all the prepared microparticles were spherical in shape. The encapsulation efficiency of GBS whole cell and supernatant microparticles were 68.07% and 63.49%, respectively; the drug loading were 5.49×108 ind · mg-1 and 3.58%, respectively; the cumulative rate of drug-release over 28 d were 64.2% and 82.8%, respectively.
-
无乳链球菌(Streptococcus agalactiae,Group B streptococcus或GBS)是一种革兰氏阳性菌,兼性厌氧,菌体呈球形或卵圆形,直径0.5~2.0 μm,成对或链状排列,无鞭毛,无芽孢,无运动性,是近年中国南方罗非鱼(Oreochromis spp.)主产区爆发大规模流行病的主要病原菌[1-2]。罗非鱼链球菌病爆发范围广,死亡率高,给罗非鱼养殖业造成巨大的经济损失。罗非鱼无乳链球菌病的主要防治措施包括减少饲料投喂、降低饲养密度、使用抗生素、水体消毒、降温以及疫苗免疫等[3]。其中疫苗免疫被认为是从根本上预防链球菌病的有效方法[4]。有报道证明无乳链球菌灭活疫苗注射免疫对罗非鱼有较好的免疫保护效果[5-7]。但由于注射疫苗操作上需耗费较多的人力与时间,且易对鱼体造成损伤,而口服疫苗可节省劳动力,同时又减轻对鱼体的损伤,适用于大规模养殖生产,因此有效的口服疫苗亟待开发。
乳酸-羟基乙酸共聚物(polylactic-co-glyconlicacid,PLGA)由乳酸和羟基乙酸无规聚合而成,它是经美国FDA认证的一种具有良好的可降解性和生物相容性的材料[8]。1979年PVEIS和LANGER用PLGA制成破伤风类毒素微球疫苗,成为第一个被世界卫生组织(WHO)批准的一次性注射疫苗[9]。PLGA微球或纳米微粒作为药物载体包埋疫苗、多肽、蛋白或小分子药物,可控制药物释放,延长药物作用时间长达数月[10-11],具有保护药物免遭破坏,降低药物毒性和刺激性以及保留蛋白的良好免疫原性等优点[12]。此外,因其可塑性PLGA在生物医学工程领域也应用于制作药物缓释载体[13]和组织工程支架等[14]。
利用PLGA作为佐剂包埋免疫原制成口服疫苗已有很多研究[15-16]。PLGA微球的粒径与口服后肠道吸收效果直接相关,一般粒径小于10 μm的微球在消化道内可以不随粪便排出体外而被肠道相关淋巴组织吸收,并通过微褶皱细胞(M cell)进入肠道集合淋巴结(Peyer′s patch);小于5 μm的微球还可以转移到脾脏等其他淋巴组织中[17-18]。鉴于破碎后的细菌成分也具有一定免疫保护作用[19-20],且更易于包埋,笔者研究探索了微球制备过程中不同条件对粒径的影响,并以合适粒径的微球包埋无乳链球菌全菌及超声破碎后的上清,分析包埋及缓释效果,为进一步开展罗非鱼口服疫苗免疫效果的研究奠定基础。
1. 材料与方法
1.1 材料
GBS菌种(S1-GDZL)由笔者实验室分离、鉴定与保存,聚乳酸-羟基乙酸(LA : GA=65 : 35,Mw 40 000~75 000 kDa,Sigma);聚乙烯醇(PVA-1788,Mw=88 000,Amresco);BCA蛋白质定量分析试剂盒(上海博彩生物科技有限公司出品);SDS-PAGE kit(MDBio,Inc);氯化钠、二氯甲烷、氢氧化钠、十二烷基硫酸钠等均为国产分析纯试剂。
1.2 方法
1.2.1 GBS灭活全菌及破碎后上清的制备
S1-GDZL菌株涂布于脑心浸出液固体培养基(BHIA),过夜培养后挑取单克隆,在28 ℃,220 r · min-1条件下培养过夜,活化菌液再转接到1 000 mL脑心浸出液液体培养基(BHI)相同条件扩大培养。
灭活GBS全菌的制备为200 mL菌液(浓度1.8×109 cfu · mL-1)中加入终浓度1%的福尔马林溶液,室温灭活72 h。离心(6 000 r · min-1,10 min)收集菌体,无菌PBS洗涤3次,最终用15 mL PBS重悬。为使GBS链断裂易于包埋,将菌液超声处理5 min(180 W,超声5 s,间隔5 s,Scientz,ⅡD,宁波新芝),超声处理前后细菌分别用革兰氏染色,10×100倍油镜观察超声处理效果,4 ℃保存备用。
GBS超声破碎后上清的制备为500 mL GBS菌液离心(6 000 r · min-1,10 min)收集菌体,灭菌PBS洗涤3次,再用20 mL灭菌PBS重悬,超声破碎1 h(400 W,超声5 s,间隔5 s),离心(8 000 r · min-1,10 min)收集上清,4 ℃保存备用。
1.2.2 复乳溶剂挥发法制备PLGA微球
参考HO等[21]的方法略作改进,一定量的PLGA溶于4 mL二氯甲烷(油相O),完全溶解后加入1 mL菌液或破碎后上清溶液(内水相W1),用涡旋振荡器高速涡旋振荡5 min(Vortex-Genie2T,Scientific Industries)形成W1/O初乳,将初乳加入到一定量的不同PVA质量浓度的水溶液[外水相W2,含50 mg · mL-1氯化钠(NaCl)]中,高速涡旋振荡8 min形成W1/O/W2复乳,复乳加入于80 mL NaCl(50 mg · mL-1)水溶液中,150 r · min-1搅拌6~8 h,使二氯甲烷挥发完全。离心微球混悬液(5 000 r · min-1,5 min)收集微球,蒸馏水洗涤3次,冷冻干燥24 h(LGJ-12型,北京松源华兴),4 ℃保存。
1.2.3 不同制备条件对微球粒径的影响
以GBS破碎后的上清为包埋对象,固定其他条件不变,观察不同PLGA质量浓度、外水相体积和PVA质量浓度对微球粒径的影响,以制备目标粒径微球。
1) 不同PLGA质量浓度对微球粒径的影响。依据上述方法,固定外水相体积为20 mL,PVA质量浓度为20 mg · mL-1,PLGA质量浓度分别为12.5 mg · mL-1、25 mg · mL-1和50 mg · mL-1制备微球。
2) 不同PVA质量浓度对微球粒径的影响。依据上述方法,固定PLGA质量浓度为25 mg · mL-1,外水相体积为20 mL,PVA质量浓度分别为20 mg · mL-1、10 mg · mL-1、5 mg · mL-1和1 mg · mL-1制备微球。
3) 不同外水相体积对微球粒径的影响。依据上述方法,固定PLGA质量浓度为25 mg · mL-1,PVA质量浓度为1 mg · mL-1,外水相体积分别为10 mL、20 mL和40 mL制备微球。
1.2.4 统计粒径分布及平均粒径并制备目标微球
参考齐琰等[22]的方法略作改进。取适量干燥后微球加水复溶,显微镜下(宁波舜宇XD30)随机选50个微球测量直径,统计粒径分布并计算平均粒径。依据不同因素对微球粒径的影响,选择合适条件制备目标微球,并以PBS作为内水相制备对照微球,用作后续试验的对照组。
1.2.5 微球包埋效果检测及外观形态观察
称取适量干燥全菌微球复溶于蒸馏水中,光学显微镜观察是否有细菌包埋入微球中。称取干燥的上清微球10 mg,加入2 mL 0.1 mol · L-1氢氧化钠(NaOH)(含50 g · L-1 SDS)水溶液,37 ℃恒温振荡24 h,离心收集上清,SDS-PAGE检测包埋效果。
分别取适量3种冻干微球粉末,均匀涂布于导电胶上,喷金后扫描电子显微镜观察微球表面形态(离子溅射仪,HITACHI E-1010;扫描电子显微镜,HITACHI,S-3400N)。
1.2.6 微球包封率和载药量的测定
参考YEH等[23]的方法略作改进,简述为精确称取全菌及上清微球各10 mg,分别加入2 mL 0.1 mol · L-1 NaOH(含50 g · L-1SDS)水溶液,37 ℃恒温振荡,使微球完全溶解。以蛋白质质量浓度为标准,用BCA微量蛋白质量浓度测定法测得制备微球时加入菌液或菌液破碎后的上清中以及微球溶解后溶解液中蛋白质质量浓度,计算全菌和上清微球的包封率和载药量。
包封率(%)=(微球溶解液中测得的蛋白质质量浓度/投入全菌或破碎后的上清中蛋白质质量浓度)×100
全菌微球载药量(菌量· mg-1)=(全菌微球包封率×投入GBS菌量)/微球质量
上清微球载药量(%)=(微球溶解液中测得的蛋白质含量/微球质量)×100
1.2.7 微球体外释放曲线的测定
参考HO等[21]的方法略作改进,简述为称取10 mg微球,加入4 mL PBS(pH 7.4)缓冲液作为缓释介质,置于37 ℃恒温震荡(150 r · min-1),分别在第1、第3、第5、第8、第18小时,第1、第2、第3、第5、第7、第9、第13、第19和第28天离心,取2 mL上清液并同时补入2 mL新鲜PBS缓冲液。上清液中的蛋白质量浓度用BCA法测定,按以下公式算出累积释放量,设3个重复。第n次累积释放量(%)= $ \frac{C_n \times 4+\sum_{i=0}^{n-1} \times C_{n-1} \times_2}{W \times X}$
Cn为第n次测得缓释介质中蛋白质浓度;Cn-1为第n-1次测得缓释介质中蛋白质质量浓度;W为投入微球的总质量;X为微球载药量。
2. 结果
2.1 不同制备条件对微球粒径的影响
2.1.1 PLGA质量浓度对微球粒径的影响
PVA质量浓度20 mg · mL-1、外水相体积20 mL条件下,随着PLGA质量浓度从12.5 mg · mL-1增加到50 mg ·mL-1,微球平均粒径从18.2 μm增加到32.1 μm(表 1),即微球平均粒径随着PLGA质量浓度的增加而变大。
表 1 乳酸-羟基乙酸共聚物浓度对微球粒径分布及平均粒径的影响Table 1 Effect of PLGA concentration on average diameter and size range of microparticlesPLGA质量浓度/mg·mL-1 PLGA concentration 外水相体积/mL outer water volume PVA质量浓度/mg·mL-1 PVA concentration 粒径范围/μm size range 平均粒径/μm average diameter 12.5 20 20 4.9~47.6 18.2 25.0 20 20 6.4~52.0 24.8 50.0 20 20 11.5~64.4 32.1 2.1.2 PVA质量浓度对微球粒径的影响
PLGA质量浓度25 mg · mL-1、外水相体积20 mL条件下,随着PVA质量浓度从20 mg · mL-1减小到1 mg ·mL-1,平均粒径从24.8 μm减小到3.8 μm(表 2),即微球平均粒径随PVA质量浓度减小而变小。
表 2 聚乙烯醇质量浓度对微球粒径分布及平均粒径的影响Table 2 Effect of PVA concentration on average diameter and size range of microparticlesPLGA质量浓度/mg·mL-1 PLGA concentration 外水相体积/mL outer water volume PVA质量浓度/mg·mL-1 PVA concentration 粒径范围/μm size range 平均粒径/μm average diameter 25 20 20 6.4~47.6 24.8 25 20 10 4.3~26.2 13.1 25 20 5 2~19.8 8.2 25 20 1 1.0~12.1 3.8 2.1.3 外水相体积对微球粒径的影响
PLGA质量浓度25 mg · mL-1,PVA质量浓度1 mg · mL-1条件下,随着外水相体积从10 mL增加到40 mL,微球平均粒径从3.2 μm增加到12.3 μm(表 3),即微球平均粒径随外水相体积增大而变大。
表 3 外水相体积对微球粒径分布及平均粒径的影响Table 3 Effect of outer water volume on average diameter and size range of microparticlesPLGA质量浓度/mg·mL-1 PLGA concentration 外水相体积/mL outer water volume PVA质量浓度/mg·mL-1 PVA concentration 粒径范围/μm size range 平均粒径/μm average diameter 25 10 1 1.2~12.6 3.2 25 20 1 1.5~14.3 3.6 25 40 1 2.2~26.9 12.3 2.2 灭活菌液超声处理后断链效果
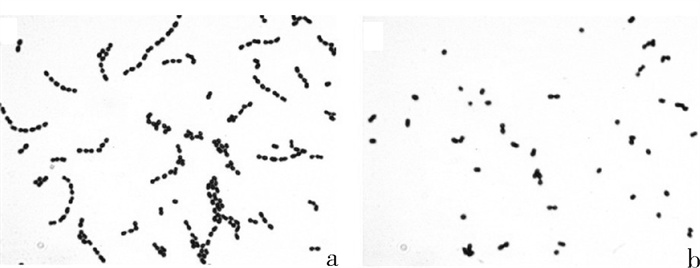
经福尔马林灭活的GBS菌液超声处理后,革兰氏染色观察可见GBS链状菌体断开,由链状分布变为球状或双球分布(图 1),这有利于包埋进PLGA微球。
2.3 目标微球的制备条件
依据PLGA质量浓度、PVA质量浓度及外水相体积对微球平均粒径的影响,选择合适的条件制备目标微球,所选条件及3种微球的粒径分布与平均粒径见表 4。
表 4 无乳链球菌全菌、破碎后上清和PBS微球的制备条件、粒径分布和平均粒径Table 4 Preparation condition, size range and average diameter of microparticles containing GBS whole cell, supernatant and PBSPLGA质量浓度/mg·mL-1 PLGA concentration 外水相体积/mL outer water volume PVA质量浓度/mg·mL-1 PVA concentration 粒径范围/μm size range 平均粒径/μm average diameter 全菌微球microparticles containing GBS whole cell 25 20 2 2.0~20.8 9.4 上清微球microparticles containing supernatant protein 25 20 1 1.0~13.8 3.9 PBS微球microparticles containing PBS 25 20 1 1.6~14.4 4.8 2.4 微球包埋效果及外观形态
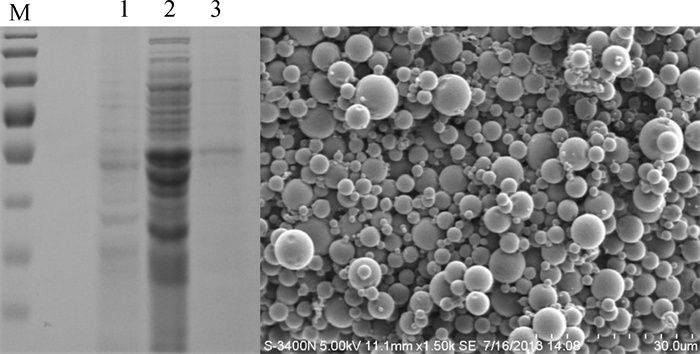
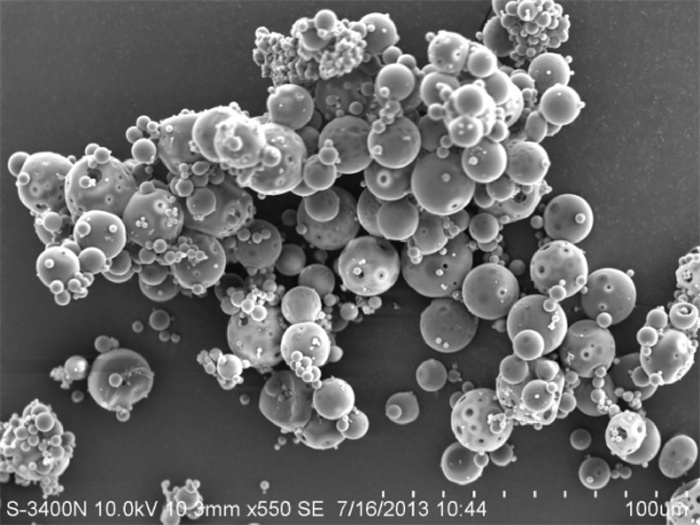
经光学显微镜观察,GBS菌体有效包埋入微球,较大粒径的微球包埋更明显;电子显微镜下微球呈球形,粒径分布相对集中,有未包埋入微球的菌体吸附在微球表面(图 2)。经SDS-PAGE检测,上清微球溶解液中有蛋白存在,说明破碎后上清也有效包埋入微球。电镜照片显示微球表面光滑无粘连,粒径分布较均匀(图 3)。PBS微球粒径分布范围较广,且较大的微球表面有小孔(图 4)。
![]() 图 3 上清微球包埋效果及电镜观察M. 蛋白标记;1. GBS破碎后沉淀;2. GBS破碎后上清;3. 上清微球溶解液Fig. 3 Embedding effect and SEM photomicrograph of microparticles containing supernatant proteinM. marker; 1. GBS sediment after ultrasonication; 2. GBS superantant after ultrasonication; 3. solution of microparticles containing supernatant
图 3 上清微球包埋效果及电镜观察M. 蛋白标记;1. GBS破碎后沉淀;2. GBS破碎后上清;3. 上清微球溶解液Fig. 3 Embedding effect and SEM photomicrograph of microparticles containing supernatant proteinM. marker; 1. GBS sediment after ultrasonication; 2. GBS superantant after ultrasonication; 3. solution of microparticles containing supernatant2.5 微球包封率和载药量
以牛血清蛋白(BSA)为标准蛋白,BCA法测得不同蛋白浓度吸光度,制作标准曲线。相同方法测得包埋前的全菌混悬液、破碎后上清溶液和包埋后2种微球溶解液中蛋白质质量浓度;比浊计测得包埋前全菌混悬液细菌浓度。根据计算公式计算出全菌微球包封率为68.07%,载药量为5.49×108个·mg-1;上清微球包封率为63.49%,载药量为3.58%。
2.6 微球体外释放曲线
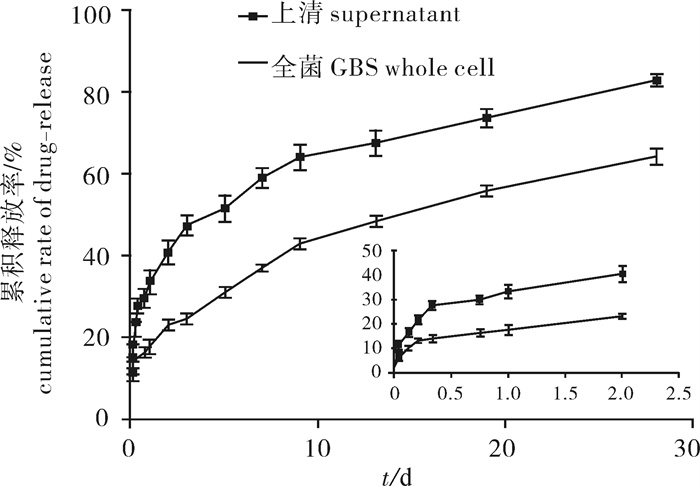
测定结果显示,2种微球所包埋药物的释放行为可分为突释和缓释2个阶段。全菌微球突释时间为前5 h,突释量13.4%,之后进入缓释阶段,最终28 d累积释放量为64.2%;而上清微球突释时间为前8 h,突释量27.7%,之后进入缓释阶段,最终28 d累积释放量高于全菌微球,达82.8%(图 5)。
3. 讨论
用PLGA包埋抗原制成微球疫苗是近年来PLGA佐剂作用的研究热点[24-26]。PLGA微球的制备有多种方法,如单一乳化法、复乳溶剂挥发法、相分离法、喷雾干燥法、溶剂扩散法等,其中复乳溶剂挥发法因包封率高且易操作而最为常用[13, 27]。复乳溶剂挥发法制备微球过程中搅拌速度、内水相和聚合物溶液的粘度、油相与外水相的体积比以及外水相PVA质量浓度对微球粒径影响较大[10, 28]。笔者从PLGA质量浓度、PVA质量浓度和外水相体积3个主要参数对微球粒径的影响进行分析。
PLGA质量浓度对微球粒径有明显的影响。笔者研究显示随着PLGA质量浓度增大,微球平均粒径明显变大,这与其他相关的研究结果相似,在有些研究中PLGA的质量浓度与微球粒径的关系甚至接近线性关系[29]。这是因为随着PLGA质量浓度的增加,初乳(即W1/O)黏度也增加,制备过程中油相和外水相界面形成粘稠的PLGA层不易扩散,从而形成较大粒径的微球。PVA质量浓度是另外一个对微球粒径影响较大的因素[30-31]。笔者研究中PVA质量浓度减小,平均粒径也随之减小。这与一些研究者的结论不一致[11, 32],YANG等[11]和ITO等[32]的试验结果显示在不同制备工艺条件下微球平均粒径随PVA质量浓度增大而减小。有观点认为PVA质量浓度对微球粒径的影响有着双向的作用:1)PVA是高分子聚合物,它能够增加复乳溶液的粘度,从而阻止乳液形成更小的液滴,使最终微球粒径变大;2)它又是乳化剂,起到稳定乳滴、阻止乳滴聚集的作用而使粒径变小[11]。所以PVA质量浓度对微球粒径的影响要看不同条件下哪一方面作用更明显。笔者试验中PVA的粘稠剂作用对微球粒径的影响更明显,高质量浓度的PVA使外水相黏性增加导致微球粒径变大。外水相体积对微球粒径也有一定影响[22-33]。笔者研究中外水相体积由10 mL增加到20 mL时微球平均粒径略有增加,而由20 mL增加到40 mL时平均粒径显著增大了近3倍。这应与外水相体积增大导致涡旋振荡的剪切力相对减小有关。因此微球制备过程中的外水相体积需要根据试验条件做出调整。
对于包埋有疫苗或药物的缓释微球来说,其降解释放行为对药效的长短有决定性作用。PLGA微球的降解速度随着PLGA分子量减小和LA/GA比例变小而增加,药物释放量也随之增加[34-35]。ROY等[36]认为微球内药物的释放速率与粒径呈负相关关系,即粒径越大,释放速率越小。因为较大粒径的微球相对表面积较小,可供药物释放的面积也较小。笔者试验中上清微球粒径小于全菌微球,释放速率大于全菌微球,与上述结论一致。但也有试验显示更大的微球药物释放更快[11, 37],SIEPMANN等[37]认为是较大粒径的微球有较多的释药通道,YANG等[11]则认为这与药物在微球内部的分布有关,低浓度的PVA可导致药物在微球内部分布不均,主要集中在外层,从而提高了其缓释速率。笔者试验以所选材料(LA : GA=65 : 35,Mw:40 000~75 000 kDa)为基础,通过改变PLGA、PVA质量浓度和外水相体积控制微球粒径以达到疫苗有效吸收和缓释的目的,所制备2种微球28 d体外累积释放量分别为64.2%和82.8%,具有较好的缓释作用。微球内部药物分布不均匀或微球表面吸附大量药物而导致的突释现象是PLGA微球缓释效果的一个瓶颈问题。控制突释有2个途径[38]:1)阻止PLGA与药物的分离,尽量除去微球溶剂;2)制作微球过程中尽量使药物与PLGA混合均匀以改变微球内部药物分布,例如改变药物盐度。因为溶剂挥发法制备微球过程中的盐析作用会影响微球的形态、物理化学性质以及药物分布[23]。用PLGA包埋霍乱弧菌(Vibrio cholerae)时发现外水相NaCl质量浓度越小,微球表面吸附的菌体就越多,突释效应也越明显[23]。YEO和PARK[39]认为整个乳液系统、聚合物和药物的交互作用、连续相(即外水相)性能均可影响微球凝固过程中孔隙的形成、药物的丢失遗漏等并最终影响到药物分布[39]。笔者试验中观察到明显的突释现象,可能与抗原在微球内分布不均有关;全菌微球突释量大于上清微球,这可能是因为全菌微球表面有未包埋进微球的菌体存留所致(图 2)。
笔者成功制备了平均粒径小于10 μm的GBS全菌、超声破碎后的上清PLGA微球,经测定包封率、载药量和缓释行为达到预期要求,为下一步研制罗非鱼无乳链球菌口服疫苗奠定了基础。
-
图 3 上清微球包埋效果及电镜观察
M. 蛋白标记;1. GBS破碎后沉淀;2. GBS破碎后上清;3. 上清微球溶解液
Figure 3. Embedding effect and SEM photomicrograph of microparticles containing supernatant protein
M. marker; 1. GBS sediment after ultrasonication; 2. GBS superantant after ultrasonication; 3. solution of microparticles containing supernatant
表 1 乳酸-羟基乙酸共聚物浓度对微球粒径分布及平均粒径的影响
Table 1 Effect of PLGA concentration on average diameter and size range of microparticles
PLGA质量浓度/mg·mL-1 PLGA concentration 外水相体积/mL outer water volume PVA质量浓度/mg·mL-1 PVA concentration 粒径范围/μm size range 平均粒径/μm average diameter 12.5 20 20 4.9~47.6 18.2 25.0 20 20 6.4~52.0 24.8 50.0 20 20 11.5~64.4 32.1 表 2 聚乙烯醇质量浓度对微球粒径分布及平均粒径的影响
Table 2 Effect of PVA concentration on average diameter and size range of microparticles
PLGA质量浓度/mg·mL-1 PLGA concentration 外水相体积/mL outer water volume PVA质量浓度/mg·mL-1 PVA concentration 粒径范围/μm size range 平均粒径/μm average diameter 25 20 20 6.4~47.6 24.8 25 20 10 4.3~26.2 13.1 25 20 5 2~19.8 8.2 25 20 1 1.0~12.1 3.8 表 3 外水相体积对微球粒径分布及平均粒径的影响
Table 3 Effect of outer water volume on average diameter and size range of microparticles
PLGA质量浓度/mg·mL-1 PLGA concentration 外水相体积/mL outer water volume PVA质量浓度/mg·mL-1 PVA concentration 粒径范围/μm size range 平均粒径/μm average diameter 25 10 1 1.2~12.6 3.2 25 20 1 1.5~14.3 3.6 25 40 1 2.2~26.9 12.3 表 4 无乳链球菌全菌、破碎后上清和PBS微球的制备条件、粒径分布和平均粒径
Table 4 Preparation condition, size range and average diameter of microparticles containing GBS whole cell, supernatant and PBS
PLGA质量浓度/mg·mL-1 PLGA concentration 外水相体积/mL outer water volume PVA质量浓度/mg·mL-1 PVA concentration 粒径范围/μm size range 平均粒径/μm average diameter 全菌微球microparticles containing GBS whole cell 25 20 2 2.0~20.8 9.4 上清微球microparticles containing supernatant protein 25 20 1 1.0~13.8 3.9 PBS微球microparticles containing PBS 25 20 1 1.6~14.4 4.8 -
[1] 卢迈新, 黎炯, 叶星, 等. 广东与海南养殖罗非鱼无乳链球菌的分离、鉴定与特性分析[J]. 微生物学通报, 2010, 37(5): 766-774. https://d.wanfangdata.com.cn/periodical/Ch9QZXJpb2RpY2FsQ0hJTmV3UzIwMjQxMTA1MTcxMzA0Eg93c3d4dGIyMDEwMDUwMjMaCGpsNDY5azVv [2] YE X, LI J, LU M X, et al. Identification and molecular typing of Streptococcus agalactiae isolated from pond-cultured tilapia in China[J]. Fish Sci, 2011, 77(4): 623-632. doi: 10.1007/s12562-011-0365-4
[3] 李安兴. 罗非鱼链球菌病防治策略新思考[J]. 海洋与渔业, 2011(9): 38-39. doi: 10.3969/j.issn.1672-4046.2011.09.028 [4] 樊海平. 罗非鱼链球菌病诊治[J]. 海洋与渔业, 2010(7): 45-46. doi: 10.3969/j.hyyyy-scqy.2010.07.018 [5] EVANS J J, KLESIUS P, SHOEMAKER C A, et al. Streptococcus agalactiae vaccination and infection stress in Nile tilapia, Oreochromis niloticus[J]. J Appl Aquac, 2005, 16(3): 105-115. doi: 10.1300/J028v16n03_07
[6] PRETTO L G, MVLLER E E, KLESIUS P, et al. Efficacy of an experimentally inactivated Streptococcus agalactiae vaccine in Nile tilapia (Oreochromis niloticus) reared in Brazil[J]. Aquac Res, 2010, 41(10): 1539-1544.
[7] 黎炯. 罗非鱼无乳链球菌的分离、鉴定及重组表面免疫原性蛋白His-Sip的免疫效果初步研究[D]. 上海: 上海海洋大学, 2011. 10.7666/d.y1947014 [8] MAKADIA H K, SIEGEL S J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier[J]. Polymers, 2011, 3(3): 1377-1397. doi: 10.3390/polym3031377
[9] 郑婷, 张月红, 陈彤, 等. 可注射缓释微球的研究进展及展望[J]. 产业与科技论坛, 2011, 10(13): 81-82. doi: 10.3969/j.issn.1673-5641.2011.13.042 [10] JEFFERY H, DAVIS S S, OHAGAN D T. The preparation and characterization of poly (lactide-co-glycolide) microparticles. 2. The entrapment of a model protein using a (water-in-oil)-in-water emulsion solvent evaporation technique[J]. Pharm Res, 1993, 10(3): 362-368. doi: 10.1023/A:1018980020506
[11] YANG Y Y, CHUNG T S, NGEE P N. Morphology, drug distribution, and in vitro release profiles of biodegradable polymeric microspheres containing protein fabricated by double-emulsion solvent extraction/evaporation method[J]. Biomaterials, 2001, 22(3): 231-241. doi: 10.1016/S0142-9612(00)00178-2
[12] 钦富华, 胡英, 高建青. 多孔PLGA微球的应用研究进展[J]. 广东药学院学报, 2012, 28(3): 1-5. doi: 10.3969/j.issn.1006-8783.2012.03.030 [13] JAIN R A. The manufacturing techniques of various drug loaded biodegradable poly (lactide-co-glycolide) (PLGA) devices[J]. Biomaterials, 2000, 21(23): 2475-2490. doi: 10.1016/S0142-9612(00)00115-0
[14] ZIMMERMANN W H, SCHNEIDERBANGER K, SCHUBERT P, et al. Tissue engineering of a differentiated cardiac muscle construct[J]. Circ Res, 2002, 90(2): 223-230. doi: 10.1161/hh0202.103644
[15] TIAN J, SUN X Q, CHEN X G, et al. The formulation and immunization of oral poly (DL-lactide-co-glycolide) microcapsules containing a plasmid vaccine against lymphocystis disease virus in Japanese flounder (Paralichthys olivaceus)[J]. Int Immunopharmacol, 2008, 8(6): 900-908. doi: 10.1016/j.intimp.2008.02.006
[16] SARTI F, PERERA G, HINTZEN F, et al. In vivo evidence of oral vaccination with PLGA nanoparticles containing the immunostimulant monophosphoryl lipid A[J]. Biomaterials, 2011, 32(16): 4052. doi: 10.1016/j.biomaterials.2011.02.011
[17] TABATA Y, INOUE Y, IKADA Y. Size effect on systemic and mucosal immune responses induced by oral administration of biodegradable microspheres[J]. Vaccine, 1996, 14(17/18): 1677-1685. doi: 10.1016/s0264-410x(96)00149-1
[18] ELDRIDGE J H, HAMMOND C J, MEULBROEK J A. Controlled vaccine release in the gut-associated lymphoid tissues. Ⅰ. Orally administered biodegradable microspheres target the peyer's patches[J]. J Control Release, 1990, 11(1/2/3): 205-214. doi: 10.1016/0168-3659(90)90133-E
[19] 王志锐, 葛新. 细菌裂解物的免疫调节作用[J]. 天津医药, 2003, 31(4): 231-234. doi: 10.3969/j.issn.0253-9896.2003.04.013 [20] 刘君, 宋晓玲, 刘莉, 等. 2株消化道优势菌对凡纳滨对虾免疫酶活性和抗白斑综合征病毒感染力的影响[J]. 水产学报, 2012, 36(3): 444-450. doi: 10.3724/SP.J.1231.2012.27592 [21] HO M L, FU Y C, WANG G J, et al. Controlled release carrier of BSA made by W/O/W emulsion method containing PLGA and hydroxyapatite[J]. J Control Release, 2008, 128(2): 142-148. doi: 10.1016/j.jconrel.2008.02.012
[22] 齐琰, 鲁莹, 钟延强. 一种包埋蛋白质药物注射用多孔微球支架制备工艺及其体外释放研究[J]. 中国药学杂志, 2008, 43(4): 291-296. doi: 10.3321/j.issn:1001-2494.2008.04.014 [23] YEH M K, CHEN J L, CHIANG C H. Vibrio cholerae-loaded poly (DLlactide co-glycolide) microparticles[J]. J Microencapsul, 2002, 19(2): 203-212. doi: 10.1080/02652040110081334
[24] BEHERA T, NANDA P K, MOHANTY C, et al. Parenteral immunization of fish, Labeo rohita with Poly d, l-lactide-co-glycolic acid (PLGA) encapsulated antigen microparticles promotes innate and adaptive immune responses[J]. Fish Shellfish Immunol, 2010, 28(2): 320-325. doi: 10.1016/j.fsi.2009.11.009
[25] ADOMAKO M, HILAIRE S, ZHENG Y, et al. Oral DNA vaccination of rainbow trout, Oncorhynchus mykiss (Walbaum), against infectious haematopoietic necrosis virus using PLGA[Poly (D, L-Lactic-Co-Glycolic Acid)] nanoparticles[J]. J Fish Dis, 2012, 35(3): 203-214. doi: 10.1111/j.1365-2761.2011.01338.x
[26] FREDRIKSEN B N, SAEVAREID K, MCAULEY L, et al. Early immune responses in Atlantic salmon (Salmo salar L) after immunization with PLGA nanoparticles loaded with a model antigen and beta-glucan[J]. Vaccine, 2011, 29(46): 8338-8349. doi: 10.1016/j.vaccine.2011.08.087
[27] 李梦雪. 聚乳酸-羟基乙酸共聚物的制备及应用[J]. 黑龙江医药, 2012, 25(3): 410-412. doi: 10.3969/j.issn.1006-2882.2012.03.034 [28] CROTTS G, PARK T G. Preparation of porous and nonporous biodegradable polymeric hollow microspheres[J]. J Control Release, 1995, 35(2): 91-105. doi: 10.1016/0168-3659(95)00010-6
[29] LI J, WANG B C, WANG Y Z, et al. Preparation and characterization of thermosensitive nanoparticles for targeted drug delivery[J]. J Macromol Sci, 2008, 45(10): 833-838. doi: 10.1080/10601320802300685
[30] YEH M K, COOMBES A G A, JENKINS P G, et al. A novel emulsification-solvent extraction technique for production of protein loaded biodegradable microparticles for vaccine and drug delivery[J]. J Control Release, 1995, 33(3): 437-445. doi: 10.1016/0168-3659(94)00123-C
[31] CELEBI N, ERDEN N, TURKYILMAZ A. The preparation and evaluation of salbutamol sulphate containing poly(lactic acid-co-glycolic acid) microspheres with factorial design-based studies[J]. Int J Pharm, 1996, 136(1): 89-100. doi: 10.1016/0378-5173(96)04491-2
[32] ITO F, FUJIMORI H, MAKINO K. Incorporation of water-soluble drugs in PLGA microspheres[J]. Colloids Surf, 2007, 54(2): 173-178. doi: 10.1016/j.colsurfb.2006.10.019
[33] 王希, 薛静, 黄岳山. 聚乳酸-羟基乙酸纳米粒的制备[J]. 中国组织工程研究, 2012, 16(3): 396-400. doi: 10.3969/j.issn.1673-8225.2012.03.003 [34] OHAGAN D T, JEFFERY H, DAVIS S S. The preparation and characterization of poly(lactide-co-glycolide) microparticles. 3. Microparticle/polymer degradation rates and the in vitro release of a model protein[J]. Int J Pharm, 1994, 103(1): 37-45. doi: 10.1016/0378-5173(94)90201-1
[35] 王明波, 冯庆玲, 佘振定, 等. PLGA微球包埋药物的稳定性及释放研究的新进展[J]. 功能材料, 2011, 42(增刊): 591-595. https://d.wanfangdata.com.cn/periodical/Ch9QZXJpb2RpY2FsQ0hJTmV3UzIwMjQxMTA1MTcxMzA0Eg1nbmNsMjAxMXo0MDAzGghsZ3lxcHhzZA%3D%3D [36] ROY S, PAL M, GUPTA B K. Indomethacin-loaded microspheres: design and preparation by a multiple-emulsification technique and their in vitro evaluation[J]. Pharm Res, 1992, 9(9): 1132-1136. doi: 10.1023/A:1015839402837
[37] SIEPMANN J, FAISANT N, AKIKI J, et al. Effect of the size of biodegradable microparticles on drug release: experiment and theory[J]. J Control Release, 2004, 96(1): 123-134. doi: 10.1016/j.jconrel.2004.01.011
[38] ALLISON S D. Analysis of initial burst in PLGA microparticles[J]. Expert Opin Drug Del, 2008, 5(6): 615-628. doi: 10.1517/17425247.5.6.615
[39] YEO Y, PARK K. Control of encapsulation efficiency and initial burst in polymeric microparticle systems[J]. Arch Pharm Res, 2004, 27(1): 1-12. doi: 10.1007/BF02980037
-
期刊类型引用(2)
1. 吴斌,樊海平,张新艳,郑磊,钟全福,张国庆,翁祖桐. 罗非鱼无乳链球菌微胶囊口服疫苗的研制及其免疫效果. 水产学报. 2016(08): 1258-1264 .  百度学术
百度学术
2. 高铭蔚,田园园,卢迈新,孙成飞,董浚键,黎宗强,叶星. 罗非鱼无乳链球菌PLGA微球口服疫苗免疫效果的研究. 免疫学杂志. 2015(02): 105-110 .  百度学术
百度学术
其他类型引用(2)



 下载:
下载:

 粤公网安备 44010502001741号
粤公网安备 44010502001741号
